Understanding Cells and Engineering Tissues
The development and function of tissues and organs are governed by intricate spatial and temporal processes that integrate genetic, mechanical, and environmental cues. At the Institute of Biological and Chemical Systems (IBCS-BIP) at Karlsruhe Institute of Technology (KIT), we investigate how these factors shape the organization and behavior of cells within three-dimensional (3D) tissues. By combining advanced imaging, mechanobiology, and interdisciplinary approaches, we aim to uncover fundamental principles of tissue development and create innovative applications in regenerative medicine and biotechnology.
Decoding Tissue Organization
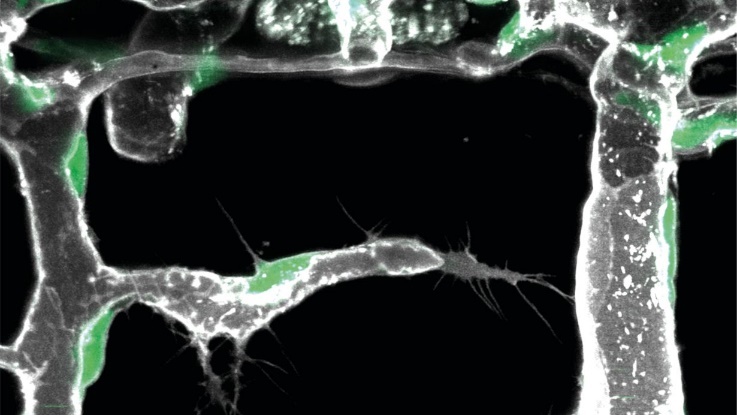
Green indicates endothelial cell nucleus, grey the outline of the blood vessels.
"Our discovery of 'pioneer cells' in organ-specific blood vessel branching reveals how unique vascular patterns develop, opening new avenues for targeted therapies in cardiovascular diseases and cancer."
Our research explores how cells self-organize into complex tissues during development. Using zebrafish and medaka as model organisms, we study the genetic and mechanical factors that guide tissue formation:
- Vascularization: We identified a novel vascular cell type, "pioneer cells," which play a critical role in organ-specific blood vessel branching. These cells respond to molecular signals from surrounding tissues, initiating vascular growth at precise locations. By decoding these molecular cues through single-cell sequencing and bioinformatics, we aim to develop therapeutic strategies for cardiovascular diseases and cancer [1].
- Scaling in Development: Through developmental quantitative trait loci (devQTL) mapping in medaka, we revealed mechanisms that link developmental timing to organismal size. This work highlights how spatial scaling during embryogenesis is controlled by distinct genetic modules governing segmentation timing and tissue size [2].
Mechanobiology: The Influence of Physical Forces
Mechanical forces are key regulators of tissue development and maintenance. Our mechanobiology research focuses on how cells sense and respond to their physical environment:
- Cellular Mechanics: We developed bio-metamaterials with tailored mechanical properties to study how human mesenchymal stem cells (hMSCs) respond to substrate stiffness and elasticity. Our findings demonstrate that cells can sense not only stiffness but also anisotropic properties like Poisson's ratio, influencing their behavior at the cytoskeletal level [3].
- Optofluidic Manipulations: Using light-induced flow fields (FLUCS), we have pioneered non-invasive methods to measure viscoelastic properties within living cells. This approach has revealed new insights into the mechanical properties of intracellular compartments during processes like oocyte fertilization [4].
Applications in 3D Tissue Engineering
“Integrating bio-metamaterials with optofluidic manipulation enables precise control over cell behavior in three dimensions, opening new possibilities for creating artificial tissues.”
Our findings have direct implications for engineering 3D tissues with defined properties:
- Regenerative Medicine: By understanding how vascular networks form in response to tissue-specific signals, we aim to design strategies for promoting vascularization in engineered tissues.
- Synthetic Biology: The integration of bio-metamaterials with optofluidic manipulation techniques enables precise control over cell behavior in 3D environments, opening new possibilities for creating artificial tissues [5].
Bridging Biology and Technology
Our work is deeply interdisciplinary, leveraging expertise from biology, physics, materials science, and engineering. Collaborations with partners across academia and industry allow us to translate fundamental discoveries into practical applications. By combining advanced imaging technologies with computational modeling, we are building a comprehensive understanding of how tissues form, function, and adapt.
Through these efforts, we aim not only to advance our knowledge of tissue organization but also to develop transformative solutions for challenges in medicine and biotechnology
References
[1] Parenchymal cues define Vegfa-driven venous angiogenesis by activating a sprouting competent venous endothelial subtype. Laetitia Préau, Anna Lischke, Melanie Merkel, Neslihan Oegel, Maria Weissenbruch, Andria Michael, Hongryeol Park, Dietmar Gradl, Christian Kupatt, Ferdinand le Noble. Nature Communications 15:3118 (2024)
[2] Modular control of vertebrate axis segmentation in time and space. Ali Seleit, Ian Brettell, Tomas Fitzgerald, Carina Vibe, Felix Loosli, Joachim Wittbrodt, Kiyoshi Naruse, Ewan Birney, Alexander Aulehla. EMBO Journal 43:4068-4091 2024
[3] Bio-Metamaterials for Mechano-Regulation of Mesenchymal Stem Cells. Natalie Munding, Magdalena Fladung, Yi Chen, Marc Hippler, Anthony D. Ho, Martin Wegener, Martin Bastmeyer, Motomu Tanaka. Advanced Functional Materials 34(20):2301133 2023
[4] ISO-FLUCS: symmetrization of optofluidic manipulations in quasi-isothermal micro-environments. Antonio Minopoli, Susan Wagner, Elena Erben, Weida Liao, Iliya D. Stoev, Eric Lauga, Moritz Kreysing. eLight 3:16 2023
[5] Opto-fluidically multiplexed assembly and micro-robotics. Elena Erben, Weida Liao, Antonio Minopoli, Nicola Maghelli, Eric Lauga, Moritz Kreysing. Light: Science & Applications 13:59 2024

