Cellular Stress Responses
Cellular stress signaling
The aim of our work is to investigate the effects of cellular stressors (e.g. particles, genotoxins and reactive oxygen species) on biological systems. We apply systems biology approaches (transcriptomics) and network analyses, to unravel underlying mechanisms of action. In particular, we focus on oxidative stress and DNA damage as triggers for stress-induced signal transduction. These processes are studied in both cell cultures and zebrafish models. To achieve this, we employ chemical and genetic perturbation strategies in order to investigate signal processing across various levels of biological organization, from individual cells to organs and entire organisms.
Current projects:
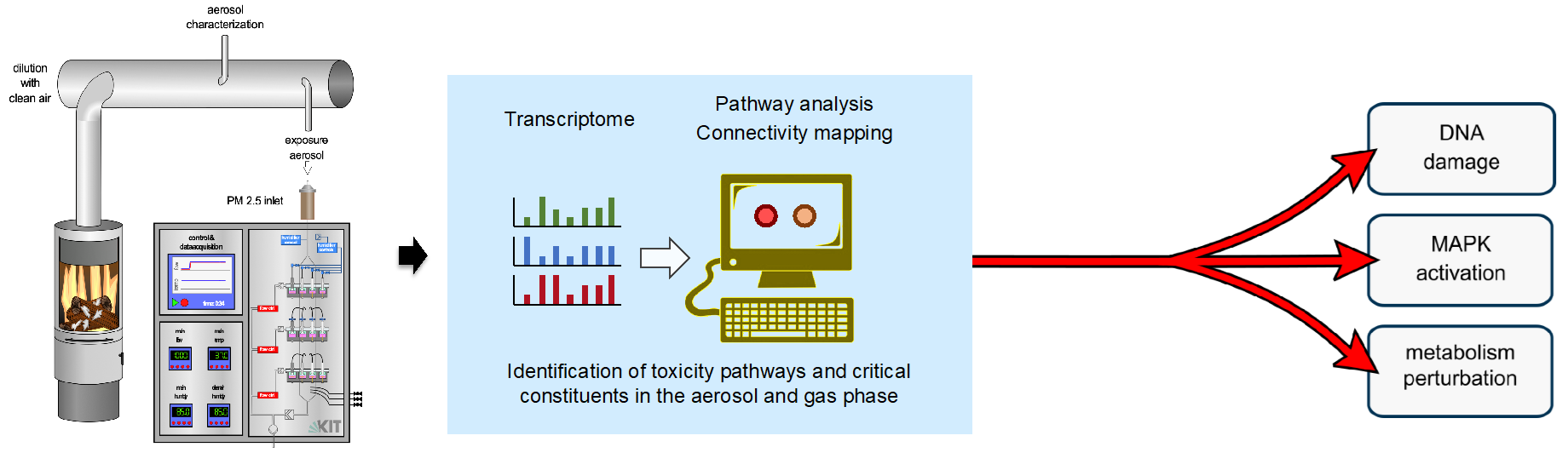
1) As part of the cross-PoF program activity ToxiDUST: Toxicity and Characterization of Health Relevant Atmospheric Fine-Dust from Emerging Sources, aerosols of different origins and compositions (including both naturally occurring and technically produced aerosols) are analyzed and evaluated with regard to their biological effects using advanced air-liquid interface exposure (ALI) systems. These projects are carried out in close collaboration with colleagues from the Institute of Technical Chemistry at KIT (https://www.itc.kit.edu/index.php). For more details, see also (https://finest-project.de/sub-projects/).
2) The ‘European Partnership for the Assessment of Risks from Chemicals’ (PARC) was developed with the overarching goal of improving knowledge about chemical substances in order to better protect human health and the environment (https://www.eu-parc.eu/). Over a seven-year period, this initiative brings together 200 organizations from across Europe. Within this framework, our group is specifically focused on investigating the mechanisms of action of non-genotoxic carcinogens.
3) As part of the EU Horizon 2020 project ‘Precision Toxicology’ (https://precisiontox.org/) we use metabolomics and transcriptomics to determine cellular responses to 200 selected chemicals (including drugs and environmental pollutants) in human cell cultures. In addition to human cell lines, he consortium utilizes a diverse range of well-established biomedical model organisms —such as fruit flies, water fleas, roundworms, and embryos of frogs and zebrafish. Artificial intelligence (AI) methods are also applied to identify molecular toxicity pathways shared across the animal kingdom. We closely collaborate with the laboratory led by Thomas Dickmeis, which conducts complementary experiments using zebrafish embryos.
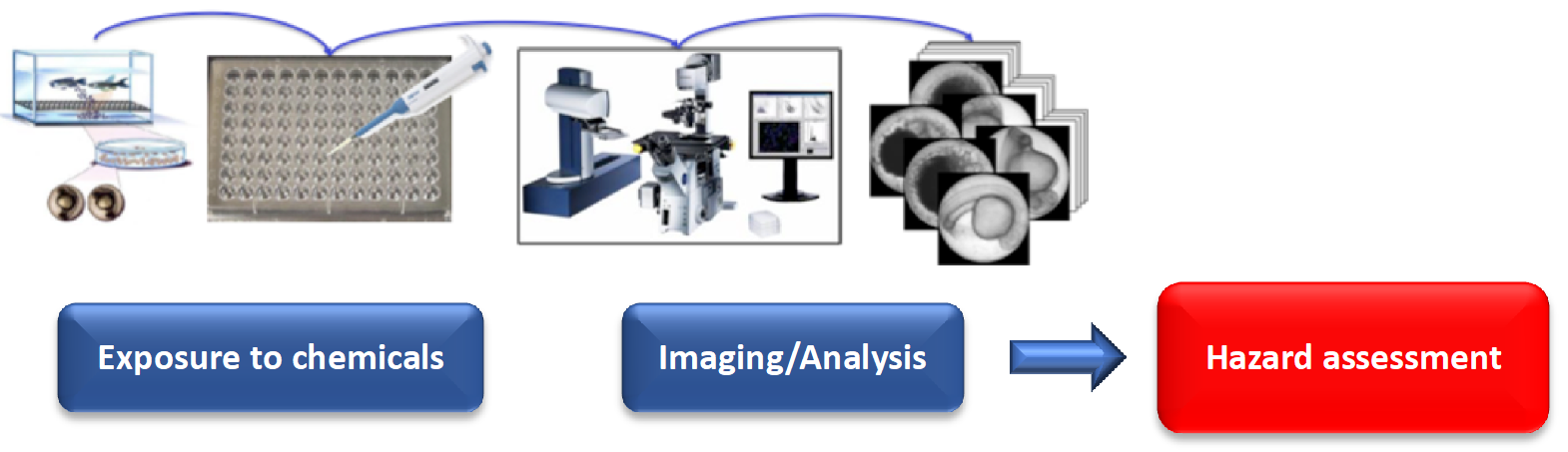
4) Zebrafish embryos are exceptionally well-suited for the systematic study of teratogenic and embryotoxic effects of chemicals. Thanks to their small size, externally developing embryos, fully sequenced genome, and the availability of numerous genetic mutants, zebrafish represent one of the most promising vertebrate models for mechanistic studies in teratology, toxicology, and toxicogenomics. Because they develop outside the maternal body, adverse effects of chemical exposure can be observed from the earliest stages of development.
Unlike in vitro studies, zebrafish embryos allow the assessment of toxic effects within a complex, intact vertebrate organism. The zebrafish facility at KIT in Karlsruhe is among the largest in the world and, together with advanced high-throughput automated microscopy systems, supports efficient large-scale toxicity screening in zebrafish embryos.
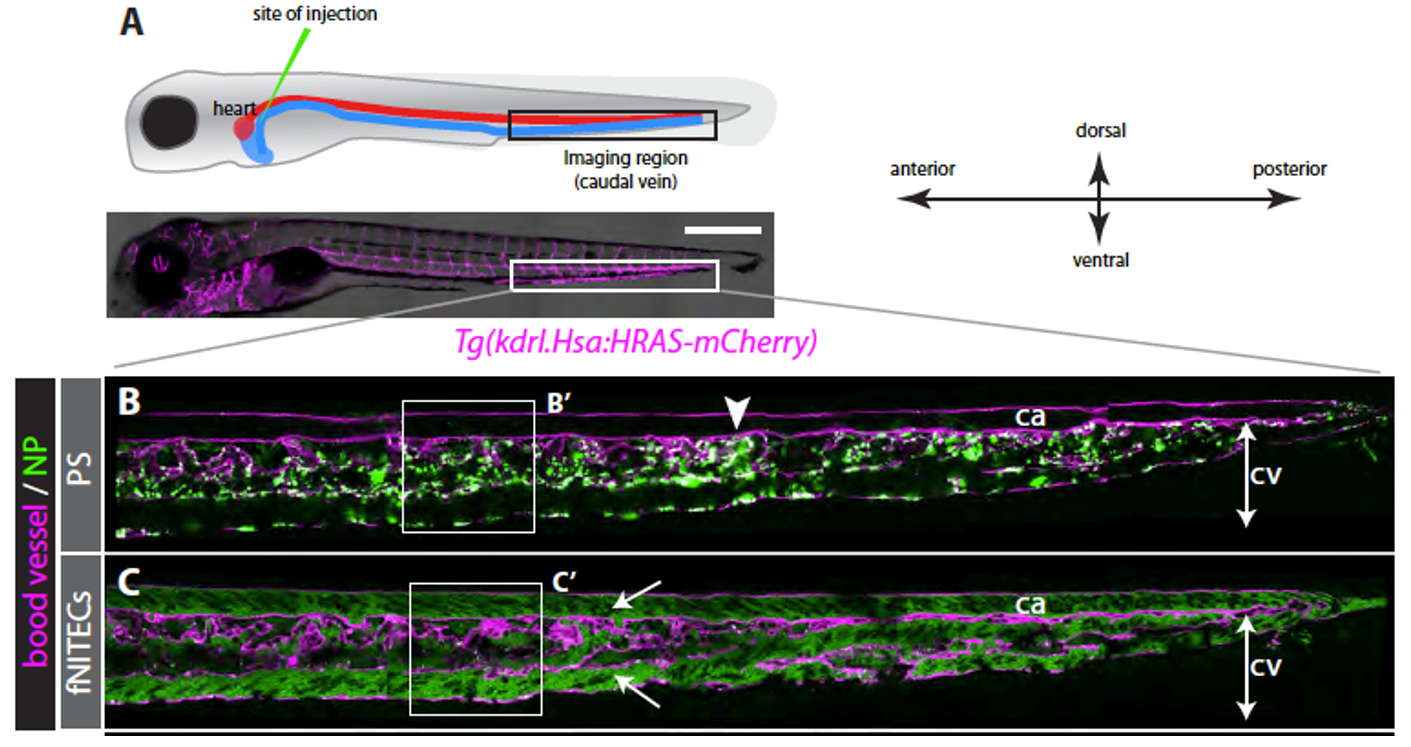
In recent years, we have established the zebrafish embryo as a model organism for studying the biodistribution and clearance of nanomaterials. This is particularly relevant in the rapidly evolving field of nanomedicine, where the mechanisms governing the distribution and elimination of nanocarriers and associated drugs remain poorly understood. Inefficient targeting and unintended scavenging of nanomedicines are considered major obstacles to their further development and clinical application.
Thanks to its optical transparency and the availability of genetic tools to label specific cell types and organs, the zebrafish embryo enables high-resolution, real-time imaging of nanomaterials in vivo. This powerful system allows us to investigate both the physicochemical properties of nanomaterials and the biological processes that influence their distribution and clearance, ultimately contributing to a deeper understanding of their kinetics and modes of action.
Dr. PD Carsten Weiss
- group leader
- Group: Weiss
- Room: CN
- Phone: +49 721 608-24906
- carsten weiss ∂does-not-exist.kit edu
- bip.ibcs.kit.edu/weiss.php
Karlsruhe Institute of Technology (KIT)
Campus North
Institute of Biological and Chemical Systems (IBCS)
Building 319
Hermann-von-Helmholtz-Platz 1
76344 Eggenstein-Leopoldshafen
Germany
Publications
Hayot, G.; Lloyd, G. R.; Diwan, G. D.; Keith, N.; Smoot, S. R.; Cramer von Clausbruch, C. A.; Kaufman, T. C.; Barnard, M.; Tindall, A. J.; Glaholt, S. P.; Massei, R.; Martínez, R.; Strähle, U.; Orsini, L.; Russell, R. B.; Tennessen, J. M.; Scholz, S.; Shaw, J. R.; Freedman, J. H.; Colbourne, J. K.; Weiss, C.; Dickmeis, T.
2025. Environmental Science & Technology, 59 (48), 25634–25648. doi:10.1021/acs.est.5c10177
Martínez, R.; González-Sánchez, J. C.; Sampani, S. I.; Scholz, S.; Escher, B. I.; Henneberger, L.; Huchthausen, J.; Whelan, M.; Dickmeis, T.; Weiss, C.; Colbourne, J. K.; Freedman, J. H.
2025. Toxicological Sciences, 208 (2), 317–329. doi:10.1093/toxsci/kfaf126
Cramer von Clausbruch, C. A.
2025, September 25. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000184934
Mülhopt, S.; Hauser, M.; Wexler, M.; Mahl, J.; Baumann, W.; Klein, S.; Diabaté, S.; Fritsch-Decker, S.; Weiss, C.; Friesen, A.; Hufnagel, M.; Hartwig A.; Gutmann, B.; Schlager, C.; Krebs, T.; Stapf, D.
2025, September 3. European Aerosol Conference (EAC 2025), Lecce, Italy, August 31–September 5, 2025
Yan, J.; Takamiya, M.; Zhang, D.; Pace, G.; Rastegar, S.; Wang, H.; Schoch, S.; Köberle, B.; Hartwig, A.; Dickmeis, T.; Weiss, C.
2025. Environment International, 197, Article no: 109349. doi:10.1016/j.envint.2025.109349
Li, H.; Scheitle, C.; Di Mauro, G.; Fuselli, S.; Fritsch-Decker, S.; Todo, T.; Weiss, C.; Vallone, D.; Lamparter, T.; Bertolucci, C.; Foulkes, N. S.
2025. Nature Communications, 16 (1), 7377. doi:10.1038/s41467-025-62795-7
Zhang, D.
2024, May 7. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000169668
Hayot, G.; Massei, R.; Lloyd, G.; Keith, N.; Diwan, G.; Martínez López, R.; Barnard, M.; Cramer von Clausbruch, C. A.; Grasse, N.; Smoot, S.; Escher, B.; Tennessen, J.; Tindall, A.; Oliver, B.; Shaw, J.; Scholz, S.; Freedman, J.; Strähle, U.; Colbourne, J. K.; Weiss, C.; Dickmeis, T.
2024. Naunyn-Schmiedeberg’s archives of pharmacology, 397 (Suppl. 1), S17–69. doi:10.1007/s00210-024-02974-3
Hayot, G.; Marcato, D.; Cramer von Clausbruch, C. A.; Pace, G.; Strähle, U.; Colbourne, J. K.; Pylatiuk, C.; Peravali, R.; Weiss, C.; Scholz, S.; Dickmeis, T.
2024. Journal of visualized experiments : JoVE, 203, Art.-Nr.: e66153. doi:10.3791/66153
Dilger, M.; Armant, O.; Ramme, L.; Mülhopt, S.; Sapcariu, S. C.; Schlager, C.; Dilger, E.; Reda, A.; Orasche, J.; Schnelle-Kreis, J.; Conlon, T. M.; Yildirim, A. Ö.; Hartwig, A.; Zimmermann, R.; Hiller, K.; Diabaté, S.; Paur, H.-R.; Weiss, C.
2023. Environment International, 179, Art.-Nr.: 108169. doi:10.1016/j.envint.2023.108169
Leibe, R.; Fritsch-Decker, S.; Gussmann, F.; Wagbo, A. M.; Wadhwani, P.; Diabaté, S.; Wenzel, W.; Ulrich, A. S.; Weiss, C.
2023. Small, 19 (34), Artkl.Nr.: 2207593. doi:10.1002/smll.202207593
Pan, M.; Solozobova, V.; Kuznik, N. C.; Jung, N.; Graessle, S.; Gourain, V.; Heneka, Y. M.; Cramer von Clausbruch, C. A.; Fuhr, O.; Munuganti, R. S. N.; Maddalo, D.; Blattner, C.; Neeb, A.; Sharp, A.; Cato, L.; Weiss, C.; Jeselsohn, R. M.; Orian-Rousseau, V.; Bräse, S.; Cato, A. C. B.
2023. Cancer Research Communications, 3 (7), 1378–1396. doi:10.1158/2767-9764.CRC-23-0111
Weiss, C.; Dickmeis, T.; Hayot, G.; Peravali, R.; Cramer von Clausbruch, C.; Pace, G.
2023. Toxicology Letters, 383, 33–42. doi:10.1016/j.toxlet.2023.05.004
Cramer von Clausbruch, C.; Weiss, C.; Dickmeis, T.; Peravali, R.; Strähle, U.; Colbourne, J.; Hayot, G.; Scholz, S.; Lopez, R. M.
2023. Naunyn-Schmiedeberg’s Archives of Pharmacology, 396 (S1), S61–62. doi:10.1007/s00210-023-02397-6
Dickmeis, T.; Hayot, G.; Clausbruch, C. C. von; Martinez Lopez, R.; Scholz, S.; Colbourne, J.; Strähle, U.; Weiss, C.; Peravali, R.
2023. Naunyn-Schmiedeberg’s Archives of Pharmacology, 396 (S1), S61
Wall, J.
2023, February 16. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000155919
Friesen, A.; Fritsch-Decker, S.; Mülhopt, S.; Quarz, C.; Mahl, J.; Baumann, W.; Hauser, M.; Wexler, M.; Schlager, C.; Gutmann, B.; Krebs, T.; Goßmann, A.-K.; Weis, F.; Hufnagel, M.; Stapf, D.; Hartwig, A.; Weiss, C.
2023. International Journal of Molecular Sciences, 24 (3), Art.-Nr.: 1927. doi:10.3390/ijms24031927
Baumann, W.; Bäger, D.; Deußen, O.; Diabaté, S.; Ellermann, N.; Emmerich, R.; Friesen, A.; Fritsch-Decker, S.; Gehrmann, H.-J.; Gries, T.; Große, A.; Guth, J.; Hartwig, A.; Hauser, M.; Hofmann, M.; Hufnagel, M.; Kehren, D.; Krug, H. F.; Kühnel, D.; Leis, J.; Mahl, J.; Marquardt, C.; Mattern, A.; Merz, D.; Möller, N.; Nau, K.; Naumann, R.; Plitzko, S.; Schlögel, K.; Stapf, D.; Steinbach, C.; Weiss, C.; Wexler, M.
2023. CFC – Carbon Fibre Cycle Carbonfasern im Kreislauf – Freisetzungsverhalten und Toxizität bei thermischer und mechanischer Behandlung - Abschlussworkshop (2023), Karlsruhe, Germany, January 19–20, 2023
Baumann, W.; Bäger, D.; Deußen, O.; Diabaté, S.; Ellermann, N.; Emmerich, R.; Friesen, A.; Fritsch-Decker, S.; Gehrmann, H. J.; Gries, T.; Große, A.; Guth, J.; Hartwig, A.; Hauser, M.; Hofmann, M.; Hufnagel, M.; Kehren, D.; Krug, H. F.; Kühnel, D.; Leis, J.; Mahl, J.; Marquardt, C.; Mattern, A.; Merz, D.; Möller, N.; Nau, K.; Naumann, R.; Plitzko, S.; Schlögel, K.; Stapf, D.; Steinbach, C.; Weiss, C.; Wexler, M.
2023
Friesen, A.
2022, November 14. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000152391
Mülhopt, S.
2022. IAC Book of Abstracts 2022, 27
Mülhopt, S.; Hauser, M.; Wexler, M.; Mahl, J.; Baumann, W.; Diabaté, S.; Fritsch-Decker, S.; Weiss, C.; Friesen, A.; Hufnagel, M.; Hartwig, A.; Gutmann, B.; Schlager, C.; Krebs, T.; Goßmann, A.-K.; Weis, F.; Stapf, D.
2022, September. 11th International Aerosol Conference (IAC 2022), Athens, Greece, September 4–9, 2022
Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Weiss, C.; Hartwig, A.
2022. International Journal of Molecular Sciences, 23 (14), 7773. doi:10.3390/ijms23147773
Mülhopt, S.
2022, July 5. 8th UFP Conference : International Symposium on Ultrafine Particles - Air Quality and Climate (2022), Brussels, Belgium, July 5–6, 2022
Mülhopt, S.; Hauser, M.; Wexler, M.; Mahl, J.; Baumann, W.; Fritsch-Decker, S.; Weiss, C.; Friesen, A.; Hufnagel, M.; Hartwig, A.; Gutmann, B.; Schlager, C.; Krebs, T.; Goßmann, A.-K.; Weis, F.; Stapf, D.
2022. 8th EFCA International Symposium on Ultrafine Particles - Air Quality and Climate. Abstract I.5, 52, European Federation of Clean Air and Environmental Protection Associations (EFCA)
Friesen, A.; Fritsch-Decker, S.; Hufnagel, M.; Mülhopt, S.; Stapf, D.; Hartwig, A.; Weiss, C.
2022. International Journal of Molecular Sciences, 23 (12), 6412. doi:10.3390/ijms23126412
Kuznik, N. C.; Solozobova, V.; Lee, I. I.; Jung, N.; Yang, L.; Nienhaus, K.; Ntim, E. A.; Rottenberg, J. T.; Muhle-Goll, C.; Kumar, A. R.; Peravali, R.; Gräßle, S.; Gourain, V.; Deville, C.; Cato, L.; Neeb, A.; Dilger, M.; Cramer von Clausbruch, C. A.; Weiss, C.; Kieffer, B.; Nienhaus, G. U.; Brown, M.; Bräse, S.; Cato, A. C. B.
2022. iScience, 25 (5), Art.Nr. 104175. doi:10.1016/j.isci.2022.104175
Weinschenk, S.; Weiss, C.; Benrath, J.; Baehr, V. von; Strowitzki, T.; Feißt, M.
2022. International Journal of Molecular Sciences, 23 (6), Article no: 3283. doi:10.3390/ijms23063283
Hansjosten, I.; Takamiya, M.; Rapp, J.; Reiner, L.; Fritsch-Decker, S.; Mattern, D.; Andraschko, S.; Anders, C.; Pace, G.; Dickmeis, T.; Peravali, R.; Rastegar, S.; Strähle, U.; Hsiao, I.-L.; Gilliland, D.; Ojea-Jimenez, I.; Ambrose, S. V. Y.; Belinga-Desaunay-Nault, M.-F. A.; Khan, A. O.; Lynch, I.; Valsami-Jones, E.; Diabaté, S.; Weiss, C.
2022. Environmental science / Nano, 91 (1), 375–392. doi:10.1039/d1en00299f
Baumann, W.; Fritsch-Decker, S.; Hauser, M.; Mahl, J.; Mülhopt, S.; Stapf, D.; Weiss, C.; Wexler, M.
2022. Karlsruher Institut für Technologie (KIT). doi:10.2314/KXP:188246253X
Mastromarco, M.; Amaducci, S.; Colonna, N.; Finocchiaro, P.; Cosentino, L.; Barbagallo, M.; Aberle, O.; Andrzejewski, J.; Audouin, L.; Bacak, M.; Balibrea, J.; Bečvář, F.; Berthoumieux, E.; Billowes, J.; Bosnar, D.; Brown, A.; Caamaño, M.; Calviño, F.; Calviani, M.; Cano-Ott, D.; Cardella, R.; Casanovas, A.; Cerutti, F.; Chen, Y. H.; Chiaveri, E.; Cortés, G.; Cortés-Giraldo, M. A.; Damone, L. A.; Diakaki, M.; Domingo-Pardo, C.; Diacono, D.; Dressler, R.; Dupont, E.; Durán, I.; Fernández-Domínguez, B.; Ferrari, A.; Ferreira, P.; Furman, V.; Göbel, K.; García, A. R.; Gawlik, A.; Gilardoni, S.; Glodariu, T.; Gonçalves, I. F.; González-Romero, E.; Griesmayer, E.; Guerrero, C.; Gunsing, F.; Harada, H.; Weiss, C.; et al.
2022. The European Physical Journal A, 58 (8), Art.-Nr.: 147. doi:10.1140/epja/s10050-022-00779-7
Murugadoss, S.; Mülhopt, S.; Diabaté, S.; Ghosh, M.; Paur, H.-R.; Stapf, D.; Weiss, C.; Hoet, P. H.
2021. Nanomaterials, 11 (12), 3226. doi:10.3390/nano11123226
Mülhopt, S.; Weiss, C.; Adler, S.; Baumann, W.; Diabaté, S.; Fritsch-Decker. Susanne; Friesen, A.; Goßmann, A.-K.; Gutmann, B.; Güttler, B.; Hauser, M.; Hartwig, A.; Hee, J.; Krebs, T.; Mahl, J.; Quicker, P.; Schlager, C.; Weis, F.; Wexler, M.; Stapf, D.
2021. "NanoCare‐Clustertreffen 2021 - Vorstellung der Projekte aus der BMBF‐Fördermaßnahme NanoCare4.0. Book of Abstracts". Hrsg. DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V
Stapf, D.; Baumann, W.; Gehrmann, H.-J.; Mülhopt, S.; Weiss, C.; Wexler, M.
2021, February 25. 4. Fachtagung Composite Recycling & LCA (2021), Online, February 25, 2021
Weiss, C.; Diabaté, S.
2021. Nanomaterials, 11 (11), Art.-Nr.: 3110. doi:10.3390/nano11113110
Huang, L. C. S.; Le, D.; Hsiao, I. L.; Fritsch-Decker, S.; Hald, C.; Huang, S.-C.; Chen, J.-K.; Hwu, J. R.; Weiss, C.; Hsu, M.-H.; Delaittre, G.
2021. Polymer chemistry, 12 (1), 50–56. doi:10.1039/d0py00710b
Diabaté, S.; Armand, L.; Murugadoss, S.; Dilger, M.; Fritsch-Decker, S.; Schlager, C.; Béal, D.; Arnal, M.-E.; Biola-Clier, M.; Ambrose, S.; Mülhopt, S.; Paur, H.-R.; Lynch, I.; Valsami-Jones, E.; Carriere, M.; Weiss, C.
2021. Nanomaterials, 11 (1), Art. Nr.: 65. doi:10.3390/nano11010065
Yan, J.; Wang, D.; Meng, Z.; Yan, S.; Teng, M.; Jia, M.; Li, R.; Tian, S.; Weiss, C.; Zhou, Z.; Zhu, W.
2021. Environmental pollution, 268 (A), Art.-Nr.: 115697. doi:10.1016/j.envpol.2020.115697
Mülhopt, S.; Hauser, M.; Wexler, M.; Baumann, W.; Diebaté, S.; Fritsch-Decker, S.; Weiss, C.; Friesen, A.; Hufnagel, M.; Hartwig. Andrea; Gutmann, B.; Schlager, C.; Krebs, T.; Goßmann, A.-K.; Weis, F.; Stapf, D.
2020. doi:10.5445/IR/1000125510
Baumann, W.; Beuchle, G.; Gehrmann, H.-J.; Hauser, M.; Merz, D.; Mülhopt, S.; Weiss, C.; Stapf, D.
2020, September 15. (M. Wexler, Ed.), 2nd Advanced Materials Online Conference (2020), Online, September 15, 2020
Mülhopt, S.; Hauser, M.; Wexler, M.; Baumann, W.; Diabaté, S.; Fritsch-Decker, S.; Weiss, C.; Friesen, A.; Hufnagel, M.; Hartwig, A.; Gutmann, B.; Schlager, C.; Krebs, T.; Goßmann, A.-K.; Weis, F.; Stapf, D.
2020, September 3. European Aerosol Conference (EAC 2020), Online, August 31–September 4, 2020
Kröll-Hermi, A.
2020, April 22. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000118578
Diabaté, S.; Fritsch-Decker, S.; Mülhopt, S.; Wexler, M.; Hauser, M.; Baumann, W.; Hufnagel, M.; Friesen, A.; Hartwig, A.; Stapf, D.; Weiss, C.
2020. Naunyn-Schmiedeberg’s archives of pharmacology, 393 (S1), S89. doi:10.1007/s00210-020-01828-y
Dietrich, C.; Weiss, C.
2020. ChemMedChem, 15 (1), 172. doi:10.1002/cmdc.201900586
Nelissen, I.; Haase, A.; Anguissola, S.; Rocks, L.; Jacobs, A.; Willems, H.; Riebeling, C.; Luch, A.; Piret, J.-P.; Toussaint, O.; Trouiller, B.; Lacroix, G.; Gutleb, A. C.; Contal, S.; Diabaté, S.; Weiss, C.; Lozano-Fernández, T.; González-Fernández, Á.; Dusinska, M.; Huk, A.; Stone, V.; Kanase, N.; Nocuń, M.; Stępnik, M.; Meschini, S.; Ammendolia, M. G.; Lewinski, N.; Riediker, M.; Venturini, M.; Benetti, F.; Topinka, J.; Brzicova, T.; Milani, S.; Rädler, J.; Salvati, A.; Dawson, K. A.
2020. Nanomaterials, 10 (8), Art.Nr. 1430. doi:10.3390/nano10081430
Weiss, C.; Carriere, M.; Fusco, L.; Capua, I.; Regla-Nava, J. A.; Pasquali, M.; Scott, J. A.; Vitale, F.; Unal, M. A.; Mattevi, C.; Bedognetti, D.; Merkoçi, A.; Tasciotti, E.; Yilmazer, A.; Gogotsi, Y.; Stellacci, F.; Delogu, L. G.
2020. ACS nano, 14 (6), 6383–6406. doi:10.1021/acsnano.0c03697
Ihantola, T.; Di Bucchianico, S.; Happo, M.; Ihalainen, M.; Uski, O.; Bauer, S.; Kuuspalo, K.; Sippula, O.; Tissari, J.; Oeder, S.; Hartikainen, A.; Rönkkö, T. J.; Martikainen, M.-V.; Huttunen, K.; Vartiainen, P.; Suhonen, H.; Kortelainen, M.; Lamberg, H.; Leskinen, A.; Sklorz, M.; Michalke, B.; Dilger, M.; Weiss, C.; Dittmar, G.; Beckers, J.; Irmler, M.; Buters, J.; Candeias, J.; Czech, H.; Yli-Pirilä, P.; Abbaszade, G.; Jakobi, G.; Orasche, J.; Schnelle-Kreis, J.; Kanashova, T.; Karg, E.; Streibel, T.; Passig, J.; Hakkarainen, H.; Jokiniemi, J.; Zimmermann, R.; Hirvonen, M.-R.; Jalava, P. I.
2020. Particle and fibre toxicology, 17 (1), Article: 27. doi:10.1186/s12989-020-00355-1
Siemer, S.; Wünsch, D.; Khamis, A.; Lu, Q.; Scherberich, A.; Filippi, M.; Krafft, M. P.; Hagemann, J.; Weiss, C.; Ding, G.-B.; Stauber, R. H.; Gribko, A.
2020. Nanomaterials, 10 (2), 383. doi:10.3390/nano10020383
Hayashi, Y.; Takamiya, M.; Jensen, P. B.; Ojea-Jiménez, I.; Claude, H.; Antony, C.; Kjaer-Sorensen, K.; Grabher, C.; Boesen, T.; Gilliland, D.; Oxvig, C.; Straehle, U.; Weiss, C.
2020. ACS nano, 14 (2), 1665–1681. doi:10.1021/acsnano.9b07233
Stapf, D.; Weiss, C.
2019, September 26. NanoCare Clustertreffen (2019), Frankfurt am Main, Germany, September 26, 2019
Stapf, D.; Baumann, W.; Hauser, M.; Mülhopt, S.; Weiss, C.; Wexler, M.
2019, September 19. Umweltbundesamt Fachaustausch: Aufbereitung und Verwertung carbonfaserhaltiger Abfälle (2019), Dessau, Germany, September 19–20, 2019
Diabaté, S.; Fritsch-Decker, S.; Marquardt, C.; Leibe, R.; Weiss, C.
2019. Toxicology letters, 314 (S1), 200. doi:10.1016/j.toxlet.2019.09.002
Murugadoss, S.; Diabaté, S.; Mülhopt, S.; Paur, H.-R.; Brassinne, F.; Mast, J.; Godderis, L.; Weiss, C.; Hoet, P.
2019, September 9. 55th Congress of the European Societies of Toxicology (EUROTOX 2019), Helsinki, Finland, September 8–11, 2019
Diabaté, S.; Fritsch-Decker, S.; Marquardt, C.; Leibe, R.; Weiss, C.
2019, September 9. 55th Congress of the European Societies of Toxicology (EUROTOX 2019), Helsinki, Finland, September 8–11, 2019
Weiss, C.
2019, September 9. 55th Congress of the European Societies of Toxicology (EUROTOX 2019), Helsinki, Finland, September 8–11, 2019
Leibe, R.; Hsiao, I.-L.; Fritsch-Decker, S.; Kielmeier, U.; Wagbo, A. M.; Voss, B.; Schmidt, A.; Hessman, S. D.; Duschl, A.; Oostingh, G. J.; Diabaté, S.; Weiss, C.
2019. Archives of toxicology, 93 (4), 871–885. doi:10.1007/s00204-019-02422-9
Mülhopt, S.; Diabaté, S.; Schlager, C.; Dilger, M.; Berger, M.; Krebs, T.; Weiss, C.; Paur, H.-R.
2019. Asian Aerosol Conference (AAC 2019), Hong Kong, Hong Kong, May 27–30, 2019
Murugadoss, S.; Diabaté, S.; Mülhopt, S.; Paur, H.-R.; Brassinne, F.; Mast, J.; Godderis, L.; Weiss, C.; Hoet, P.
2019. Toxicology letters, 314 (S1), 207
Weiss, C.
2019. Toxicology letters, 314 (S1), 34
Fritsch-Decker, S.; An, Z.; Yan, J.; Hansjosten, I.; Al-Rawi, M.; Peravali, R.; Diabaté, S.; Weiss, C.
2019. Nanomaterials, 9 (8), 1172. doi:10.3390/nano9081172
Mülhopt, S.; Diabaté, S.; Schlager, C.; Berger, M.; Dilger, M.; Krebs, T.; Paur, H.-R.; Weiss, C.; Stapf, D.
2019. 7th UFP Conference : International Symposium on Ultrafine Particles - Air Quality and Climate (2019), Brussels, Belgium, May 15–16, 2019
Le, D.; Wagner, F.; Takamiya, M.; Hsiao, I.-L.; Gil Alvaradejo, G.; Strähle, U.; Weiss, C.; Delaittre, G.
2019. Chemical communications, 55 (26), 3741–3744. doi:10.1039/c9cc00407f
Hsiao, I.-L.; Fritsch-Decker, S.; Leidner, A.; Al-Rawi, M.; Hug, V.; Diabaté, S.; Grage, S. L.; Meffert, M.; Stoeger, T.; Gerthsen, D.; Ulrich, A. S.; Niemeyer, C. M.; Weiss, C.
2019. Small, 15 (10), 1805400. doi:10.1002/smll.201805400
Le, D.; Dilger, M.; Pertici, V.; Diabaté, S.; Gigmes, D.; Weiss, C.; Delaittre, G.
2019. Angewandte Chemie / International edition, 58 (14), 4725–4731. doi:10.1002/anie.201813434
Kowoll, T.; Fritsch-Decker, S.; Diabaté, S.; Nienhaus, G. U.; Gerthsen, D.; Weiss, C.
2018. Journal of nanobiotechnology, 16 (1), Article no 100. doi:10.1186/s12951-018-0426-2
Mane, S. R.; Hsiao, I.-L.; Takamiya, M.; Le, D.; Straehle, U.; Barner-Kowollik, C.; Weiss, C.; Delaittre, G.
2018, September 6. doi:10.1002/smll.201870162
Kanashova, T.; Sippula, O.; Oeder, S.; Streibel, T.; Passig, J.; Czech, H.; Kaoma, T.; Sapcariu, S. C.; Dilger, M.; Paur, H.-R.; Schlager, C.; Mülhopt, S.; Weiss, C.; Schmidt-Weber, C.; Traidl-Hoffmann, C.; Michalke, B.; Krebs, T.; Karg, E.; Jakobi, G.; Scholtes, S.; Schnelle-Kreis, J.; Sklorz, M.; Orasche, J.; Müller, L.; Reda, A.; Rüger, C.; Neumann, A.; Abbaszade, G.; Radischat, C.; Hiller, K.; Grigonyte, J.; Kortelainen, M.; Kuuspalo, K.; Lamberg, H.; Leskinen, J.; Nuutinen, I.; Torvela, T.; Tissari, J.; Jalava, P.; Kasurinen, S.; Uski, O.; Hirvonen, M.-R.; Buters, J.; Dittmar, G.; Jokiniemi, J. K.; Zimmermann, R.
2018. Journal of Molecular and Clinical Medicine, 1 (1), 23–35. doi:10.31083/j.jmcm.2018.01.004
Hsiao, I.-L.; Fritsch-Decker, S.; Al-Rawi, M.; Leidner, A.; Grage, S. L.; Ulrich, A. S.; Niemeyer, C. M.; Weiss, C.
2018. International Conference on Nanotoxicology (NanoTox 2018), Neuss, Germany, August 18–September 21, 2018
Hansjosten, I.; Diabaté, S.; Weiss, C.; Lynch, I.; Valsami-Jones, E.
2018. 9th International Conference on Nanotoxicology (NanoTox 2018), Neuss, Germany, September 18–21, 2018
Weiss, C.
2018. International Symposium ’Debugging NanoBio-Interfaces to Promote Clinical Translation (2018), Mainz, Germany, August 23–25, 2018
Mane, S. R.; Hsiao, I.-L.; Takamiya, M.; Le, D.; Straehle, U.; Barner-Kowollik, C.; Weiss, C.; Delaittre, G.
2018. Small, 14 (36), 1801571/1–8. doi:10.1002/smll.201801571
Fritsch-Decker, S.; Marquardt, C.; Stoeger, T.; Diabaté, S.; Weiss, C.
2018. Archives of toxicology, 92 (7), 2163–2174. doi:10.1007/s00204-018-2223-y
Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; Gómez de la Torre, J.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; Lynch, I.; Mahon, E.; Nelissen, I.; Piella, J.; Puntes, V.; Ray, S.; Schneider, R.; Wilkins, T.; Weiss, C.; Paur, H.-R.
2018. Nanomaterials, 8 (5), Article: 311. doi:10.3390/nano8050311
Dilger, M.; Ramme, L.; Sapcariu, S. C.; Mülhopt, S.; Schlager, C.; Reda, A.; Orasche, J.; Armant, O.; Maser, E.; Hartwig, A.; Zimmermann, R.; Hiller, K.; Diabate, S.; Paur, H. R.; Weiss, C.
2018. 26. Analytica : Internationale Leitmesse für Labortechnik, Analytik, Biotechnologie und analytica Conference (2018), Munich, Germany, April 10–13, 2018
Oeder, S.; Candeias, J.; Kanashova, T.; Sapcariu, S.; Richthammer, P.; Stengel, B.; Dilger, M.; Murugadoss, S.; Sippula, O.; Streibel, T.; Sklorz, M.; Orasche, J.; Ulbrich, A.; Miersch, T.; Czech, H.; Rüger, C.; Schwemer, T.; Harndorf, H.; Buchholz, B.; Paur, H.; Weiss, C.; Jokiniemi, J.; Hirvonen, M. R.; Hiller, K.; Dittmar, G.; Schmidt-Weber, C.; Buters, J. T. M.; Zimmmermann, R.
2018. 26. Analytica : Internationale Leitmesse für Labortechnik, Analytik, Biotechnologie und analytica Conference (2018), Munich, Germany, April 10–13, 2018
Lacroix, G.; Koch, W.; Ritter, D.; Gutleb, A. C.; Larsen, S. T.; Loret, T.; Zanetti, F.; Constant, S.; Chortarea, S.; Rothen-Rutishauser, B.; Hiemstra, P. S.; Frejafon, E.; Hubert, P.; Gribaldo, L.; Kearns, P.; Aublant, J.-M.; Diabaté, S.; Weiss, C.; Groot, A. de; Kooter, I.
2018. Applied In Vitro Toxicology, 4 (2), 1–16. doi:10.1089/aivt.2017.0034
Hansjosten, I.; Rapp, J.; Reiner, L.; Vatter, R.; Fritsch-Decker, S.; Peravali, R.; Palosaari, T.; Joossens, E.; Gerloff, K.; Macko, P.; Whelan, M.; Gilliland, D.; Ojea-Jimenez, I.; Monopoli, M. P.; Rocks, L.; Garry, D.; Dawson, K.; Röttgermann, P. J. F.; Murschhauser, A.; Rädler, J. O.; Tang, S. V. Y.; Gooden, P.; Belinga-Desaunay, M.-F. A.; Khan, A. O.; Briffa, S.; Guggenheim, E.; Papadiamantis, A.; Lynch, I.; Valsami-Jones, E.; Diabaté, S.; Weiss, C.
2018. Archives of toxicology, 92 (2), 633–649. doi:10.1007/s00204-017-2106-7
Dilger, M.
2018. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000065811
Mülhopt, S.; Diabate, S.; Schlager, C.; Marco, D.; Berger, M.; Krebs, T.; Weiss, C.; Paur, H.-R.
2017. Nanosafety 2017, Saarbrücken, October 11-13, 2017
Mülhopt, S.; Diabate, S.; Schlager, C.; Marco, D.; Berger, M.; Krebs, T.; Weiss, C.; Paur, H.-R.
2017. Nanosafety 2017, Saarbrücken, October 11-13, 2017. Hrsg.: E. Arzt, 58, Leibniz-Institut für Neue Medien
Mülhopt, S.; Diabate, S.; Schlager, C.; Marco, D.; Berger, M.; Krebs, T.; Weiss, C.; Paur, H.-R.
2017. Journal of aerosol medicine and pulmonary drug delivery, 30 (3), A-1-A-38
Mülhopt, S.; Krebs, T.; Schlager, C.; Dilger, M.; Weiss, C.; Diabaté, S.; Paur, H.-R.
2017. 3rd International Conference on Toxicological Alternatives & Translational Toxicology 2017, Nanjing, China, 9th - 12th July 2017
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Huber, A.; Paur, H.; Mülhopt, S.; Dilger, M.; Weiß, C.; Buchholz, B.; Stengel, B.; Hiller, K.; Sapccariu, S. C.; Berube, K. A.; Wlodarcyzk, A. J.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Klingbeil, S.; Orasche, J.; Müller, L.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Smita, S.; Orasche, J.; Uski, O.; Jalava, P.; Happo, M.; Hartikainen, A.; Lamberg, H.; Hirvonen, M.-R.; Kasurinen, S.; Sippula, O.; Jokiniemi, J.
2017. European Aerosol Conference (EAC 2017), Zurich, Switzerland, 27th August - 1st September 2017
Dilger, M.; Orasche, J.; Schlager, C.; Mülhopt, S.; Zimmermann, R.; Paur, H.-R.; Diabaté, S.; Weiss, C.
2017. European Aerosol Conference 2017, Zürich, Schweiz, 27.08.-01.09.2017
Hansjosten, I.
2017. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000073414
Landgraf, L.; Nordmeyer, D.; Schmiel, P.; Gao, Q.; Ritz, S.; Gebauer, J. S.; Graß, S.; Diabaté, S.; Treuel, L.; Graf, C.; Rühl, E.; Landfester, K.; Mailänder, V.; Weiss, C.; Zellner, R.; Hilger, I.
2017. Scientific reports, 7, 4341. doi:10.1038/s41598-017-02958-9
Mülhopt, S.; Diabate, S.; Schlager, C.; Dilger, M.; Weiss, C.; Krebs, T.; Paur, H.-R.
2017. 6th International Symposium on Ultrafine Particles Air Quality and Climate, Brussels, B, May 10-11, 2017
Hansjosten, I.; Volkmann, K.; Rapp, J.; Reiner, L.; Vatter, R.; Palosaari, T.; Joossens, E.; Gerloff, K.-B.; Whelan, M.; Gilliland, D.; Ojea-Jimenez, I.; Monopoli, M. P.; Rocks, L.; Garry, D.; Dawson, K.; Röttgermann, P. J.; Murschhauser, A.; Rädler, J. O.; Tang, S. V. Y.; Gooden, P.; Lynch, I.; Valsami-Jones, E.; Diabate, S.; Weiss, C.
2017. New Tools and Approaches for Nanomaterials Safety Assessment, Malaga, E, February 7-9, 2017
Kowoll, T.; Müller, E.; Fritsch-Decker, S.; Hettler, S.; Störmer, H.; Weiss, C.; Gerthsen, D.
2017. Scanning, 2017, Article ID 4907457. doi:10.1155/2017/4907457
Hayashi, Y.; Miclaus, T.; Murugadoss, S.; Takamiya, M.; Scavenius, C.; Kjaer-Sorensen, K.; Enghild, J. J.; Strähle, U.; Oxvig, C.; Weiss, C.; Sutherland, D. S.
2017. Environmental science / Nano, 4 (4), 895–906. doi:10.1039/C7EN00071E
Wittig, A.; Gehrke, H.; Favero, G. D.; Fritz, E.-M.; Al-Rawi, M.; Diabaté, S.; Weiss, C.; Sami, H.; Ogris, M.; Marko, D.
2017. Nanomaterials, 7 (1), Art. Nr.: 18. doi:10.3390/nano7010018
Mülhopt, S.; Diabaté, S.; Schlager, C.; Dilger, M.; Murugadoss, S.; Weiss, C.; Krebs, T.; Tang, S.; Gooden, P.; Koekemoer, L.-A.; Dekkers, S.; Cassee, F.; Paur, H.-R.
2017. New Tools and Approaches for Nanomaterial Safety Assessment, Malaga, Spain, February 7 – 9, 2017
Mülhopt, S.; Diabaté, S.; Schlager, C.; Dilger, M.; Murugadoss, S.; Weiss, C.; Krebs, T.; Tang, S.; Gooden, P.; Koekemoer, L.-A.; Dekkers, S.; Cassee, F.; Paur, H.-R.
2017. New Tools and Approaches for Nanomaterial Safety Assessment, Malaga, Spain, February 7 – 9, 2017, 205–206
Marquardt, C.; Fritsch-Decker, S.; Al-Rawi, M.; Diabaté, S.; Weiss, C.
2017. Toxicology, 379, 40–47. doi:10.1016/j.tox.2017.01.019
Dilger, M.; Orasche, J.; Zimmermann, R.; Paur, H.-R.; Diabat{\’e}, S.; Weiss, C.
2016. Archives of toxicology, 90 (12), 3029–3044. doi:10.1007/s00204-016-1659-1
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodarcyzk, A. J.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.; Smita, S.; Orasche, J.; Torvela, T.; Titta, P.; Kortelainen, M.; Lamberg, H.; Tissari, J.; Leskinen, A.; Jalava, P.; Hirvonen, M. R.; Kasurinen, S.; Jalava, P.; et al.
2016. 22nd European Aerosol Conference (EAC), Tours, F, September 4-9, 2016. Proceedings on USB-Stick
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodarcyzk, A. J.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.; Smita, S.; Orasche, J.; Torvela, T.; Titta, P.; Kortelainen, M.; Lamberg, H.; Tissari, J.; Leskinen, A.; Jalava, P.; Hirvonen, M. R.; Kasurinen, S.; Jalava, P.; et al.
2016. 22nd European Aerosol Conference (EAC 2016), Tours, France, September 4–9, 2016
Mülhopt, S.; Dilger, M.; Diabate, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Weiss, C.; Paur, H. R.
2016. 11th International Particle Toxicology Conference (IPTC 2016), Singapore, SGP, September 26-30, 2016
Mülhopt, S.; Diabate, S.; Schlager, C.; Murugadoss, S.; Dilger, M.; Weiss, C.; Krebs, T.; Tang, S.; Gooden, P.; Paur, H. R.
2016. 22nd European Aerosol Conference (EAC), Tours, F, September 4-9, 2016.Proceedings on USB-Stick
Mülhopt, S.; Diabate, S.; Schlager, C.; Murugadoss, S.; Dilger, M.; Weiss, C.; Krebs, T.; Tang, S.; Gooden, P.; Paur, H. R.
2016. 22nd European Aerosol Conference (EAC 2016), Tours, France, September 4–9, 2016
Sapcariu, S. C.; Kanashova, T.; Dilger, M.; Diabaté, S.; Oeder, S.; Passig, J.; Radischat, C.; Buters, J.; Sippula, O.; Streibel, T.; Paur, H.-R.; Schlager, C.; Mülhopt, S.; Stengel, B.; Rabe, R.; Harndorf, H.; Krebs, T.; Karg, E.; Gröger, T.; Weiss, C.; Dittmar, G.; Hiller, K.; Zimmermann, R.
2016. PLoS one, 11 (6), e0157964. doi:10.1371/journal.pone.0157964
Mülhopt, S.; Diabate, S.; Schlager, C.; Murugadoss, S.; Dilger, M.; Weiss, C.; Krebs, T.; Tang, S.; Gooden, P.; Paur, H. R.
2016. Air-Liquid Interface Workshop, Paris, March 21, 2016
Mülhopt, S.; Dilger, M.; Diabate, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Waescher, T.; Weiss, C.; Paur, H.-R.
2016. Journal of Aerosol Science, 96, 38–55. doi:10.1016/j.jaerosci.2016.02.005
Schreck, I.; Grico, N.; Hansjosten, I.; Marquardt, C.; Bormann, S.; Seidel, A.; Kvietkova, D. L.; Pieniazek, D.; Segerbäck, D.; Diabate, S.; Van Der Horst, G. T. J.; Oesch-Bartlomowicz, B.; Oesch, F.; Weiss, C.
2016. Oncogene, 35 (7), 908–918. doi:10.1038/onc.2015.145
Pahlke, G.; Tiessen, C.; Domnanich, K.; Kahle, N.; Groh, I. A. M.; Schreck, I.; Weiss, C.; Marko, D.
2016. Toxicology Letters, 240 (1), 93–104. doi:10.1016/j.toxlet.2015.10.003
Schweitzer, B.
2015. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000050561
Mülhopt, S.; Diabate, S.; Schlager, C.; Dilger, M.; Krebs, T.; Weiss, C.; Paur, H. R.
2015. Fachtagung Neue Entwicklungen bei der Messung und Beurteilung der Luftqualität, Nürnberg, 20.-21.Oktober 2015
Mülhopt, S.; Diabate, S.; Schlager, C.; Dilger, M.; Krebs, T.; Weiss, C.; Paur, H. R.
2015. Neue Entwicklungen bei der Messung und Beurteilung der Luftqualität : Nürnberg, 20. und 21.Oktober 2015, 243–247, VDI Verlag
Mülhopt, S.; Diabate, S.; Schlager, C.; Murugadoss, S.; Dilger, M.; Weiss, C.; Krebs, T.; Paur, H. R.
2015. European Aerosol Conference 2015 (EAC 2015), Milano, I, September 6-11, 2015
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hiller, K.; Sapccariu, S. C.; BerBe, K. A.; Wlodarcyzk, A. J.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.; Smita, S.; Orasche, J.; Torvela, T.; Tiitta, P.; Yli-Pirilä, P.; Kortelainen, M.; Leskinen, J.; Lamberg, H.; Grigonyte, J.; Tissari, J.; Leskinen, A.; Kuuspalo, K.; et al.
2015. European Aerosol Conference 2015 (EAC 2015), Milano, I, September 6-11, 2015
Paur, H. R.; Mahon, E.; Lynch, I.; Bergfeldt, T.; Diabate, S.; Forsgren, J.; Mülhopt, S.; Bagaria, J. P.; Puntes, V.; Schneider, R.; Weiss, C.; Wilkins, T.; Jiang, Y.
2015. QualityNano Conference, Heraklion, GR, July 15-17, 2015
Mülhopt, S.; Dilger, M.; Diabate, S.; Schlager, C.; Krebs, T.; Weiss, C.; Paur, H. R.
2015. EuroNanoForum 2015, Riga, LV, June 10-12, 2015
Mülhopt, S.; Dilger, M.; Schlager, C.; Krebs, T.; Diabaté, S.; Weiss, C.; Paur, H.-R.
2015. Energy, Science and Technology, Conference and Exhibition (EST 2015), Karlsruhe, Germany, May 20–22, 2015
Oeder, S.; Kanashova, T.; Sippula, O.; Sapcariu, S. C.; Streibel, T.; Arteaga-Salas, J. M.; Passig, J.; Dilger, M.; Paur, H. R.; Schlager, C.; Mülhopt, S.; Diabate, S.; Weiss, C.; Stengel, B.; Rabe, R.; Harndorf, H.; Torvela, T.; Jokiniemi, J. K.; Hirvonen, M. R.; Schmidt-Weber, C.; Traidl-Hoffmann, C.; BeruBe, K. A.; Wlodarczyk, J.; Prytherch, Z.; Michalke, B.; Krebs, T.; Prevot, A. S. H.; Kelbg, M.; Tiggesbäumker, J.; Karg, E.; Jakobi, G.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Matuschek, G.; Sklorz, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Elsasser, M.; Reda, A.; Gröger, T.; Weggler, B.; Czech, H.; Rüger, C. P.; Abbaszade, G.; Radischat, C.; Hiller, K.; Buters, J. T. M.; et al.
2015. PLoS one, 10 (6), e126536. doi:10.1371/journal.pone.0126536
Mülhopt, S.; Dilger, M.; Schlager, C.; Krebs, T.; Diabate, S.; Weiss, C.; Paur, H. R.
2015. EST 2015 - Energy, Science and Technology, Karlsruhe, May 20-22, 2015
Mülhopt, S.; Diabate, S.; Schlager, C.; Dilger, M.; Weiss, C.; Krebs, T.; Paur, H.
2015. 20th International Congress on Aerosols in Medicine and Pulmonary Drug Delivery (ISAM 2015), München, May 30 - June 3, 2015 Journal of Aerosol Medicine and Pulmonary Drug Delivery, 28( 2015) No.3, A30 (Abstract)
Panas, A.; Comouth, A.; Saathoff, H.; Leisner, T.; Al-Rawi, M.; Simon, M.; Seemann, G.; Dössel, O.; Mülhopt, S.; Paur, H.-R.; Fritsch-Decker, S.; Weiss, C.; Diabaté, S.
2014. Beilstein journal of nanotechnology, 5 (1), 1590–1602. doi:10.3762/bjnano.5.171
Armand, L.; Biola-Clier, M.; Dilger, M.; Mülhopt, S.; Schlager, C.; Paur, H. R.; Collin-Faure, V.; Diabate, S.; Weiss, C.; Herlin-Biome, N.; Rabilloud, T.; Carriere, M.
2014. 4th International Conference on Safe Production and Use of Nanomaterials (NANOSAFE), Grenoble, F, November 18-20, 2014
Mülhopt, S.; Dilger, M.; Schlager, C.; Zimmermann, R.; Diabate, S.; Weiss, C.; Paur, H. R.
2014. Conference on Aerosol Technology, Karlsruhe, June 16-18, 2014 Book of Abstracts T260A02
Mülhopt, S.; Dilger, M.; Schlager, C.; Diabate, S.; Braakhuis, H.; Weiss, C.; Paur, H. R.
2014. 7th International Nanotoxicology Congress (NanoTox 2014), Antalya, TR, April 23- 26, 2014 Book of Abstracts
Mülhopt, S.; Paur, H. R.; Adelhelm, C.; Diabate, S.; Forsgren, J.; Mahon, E.; Bagaria, J. P.; Puntes, V.; Schneider, R.; Wilkins, T.; Jiang, Y.; Weiss, C.
2014. 7th International Nanotoxicology Congress (NanoTox 2014), Antalya, TR, April 23- 26, 2014 Book of Abstracts
Gebel, T.; Foth, H.; Damm, G.; Freyberger, A.; Kramer, P. J.; Lilienblum, W.; Röhl, C.; Schupp, T.; Weiss, C.; Wollin, K. M.; Hengstler, J. G.
2014. Archives of toxicology, 88, 2191–2211. doi:10.1007/s00204-014-1383-7
Simon, M.; Wülfers, E. M.; Tavernier, A.; Fritsch-Decker, S.; Müller, E.; Seiter, J.; Weiss, C.; Dössel, O.; Gerthsen, D.; Seemann, G.
2014. Biomedizinische Technik / Biomedical Engineering, 59 (Suppl.1), S510-S513
Schnelle-Kreis, J.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hirvonen, M. R.; Jokiniemi, J.; Torvela, T.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodardcyzk, A. J.; Sippula, O.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Lintelmann, J.; Matuschek, G.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Elsasser, M.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.; Zimmermann, R.
2014. 2014 International Aerosol Conference (IAC 2014), Busan, Korea, August 28 - September 2, 2014
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hirvonen, M. R.; Jokiniemi, J.; Torvela, T.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodarcyzk, A. J.; Sippula, O.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Matuschek, G.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Elasasser, M.; Rheda, A.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.
2014. 2014 International Aerosol Conference (IAC 2014), Busan, Korea, August 28 - September 2, 2014
Volkmann, K.; Hansjosten, I.; Lynch, I.; Valsami Jones, E.; Diabate, S.; Weiss, C.
2014. descriptors as a basis for safer-by-design approaches. 7th International Nanotoxicology Congress (NanoTox 2014), Antalya, TR, April 23- 26, 2014 Book of Abstracts
Lynch, I.; Weiss, C.; Valsami-Jones, E.
2014. Nano today, 9, 266–270. doi:10.1016/j.nantod.2014.05.001
Khan, A.; Weiss, C.; Schweitzer, B.; Hansjosten, I.; Mikut, R.; Reischl, M.
2014. BMT 2014 : 48.Jahrestagung der Deutschen Gesellschaft für Biomedizinische Technik (DGBMT), Hannover, 8.-10.Oktober 2014
Khan, A.; Weiss, C.; Schweitzer, B.; Hansjosten, I.; Mikut, R.; Reischl, M.
2014. Biomedizinische Technik / Biomedical Engineering, 59 (Suppl.1), S518-S521
Lynch, I.; Weiss, C.; Valsami-Jones, E.
2014. 7th International Nanotoxicology Congress (NanoTox 2014), Antalya, TR, April 23- 26, 2014 Book of Abstracts
Deschamps, E.; Weidler, P. G.; Friedrich, F.; Weiss, C.; Diabate, S.
2014. Environmental geochemistry and health, 36, 225–233. doi:10.1007/s10653-013-9560-9
Mülhopt, S.; Dilger, M.; Schlager, C.; Zimmermann, R.; Diabate, S.; Weiss, C.; Paur, H. R.
2013. European Aerosol Conference (EAC 2013), Praha, CZ, September 1-6, 2013
Paur, H. R.; Mülhopt, S.; Dilger, M.; Schlager, C.; Krebs, T.; Zimmermann, R.; Diabate, S.; Weiss, C.
2013. European Aerosol Conference (EAC 2013), Praha, CZ, September 1-6, 2013
Orasche, J.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiß, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodarcyzk, A. J.; Sippula, O.; Jokiniemi, J.; Hirvonen, M. R.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Abbaszade, G.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Areaga Salas, M.; Klingbeil, S.; Richthammer, P.; Müller, L.; Elsasser, M.; Rheda, A.; Werner, B.; Passig, J.; Gröger, T.; Radischat, C.; Zimmermann, R.
2013. European Aerosol Conference (EAC 2013), Praha, CZ, September 1-6, 2013
Paur, H. R.; Adelhelm, C.; Diabate, S.; Forsgren, J.; Mahon, E.; Mülhopt, S.; Bagaria, J. P.; Puntes, V.; Schneider, R.; Weiss, C.; Wilkins, T.; Jiang, Y.
2013. European Aerosol Conference (EAC 2013), Praha, CZ, September 1-6, 2013
Gehrke, H.; Frühmesser, A.; Pelka, J.; Esselen, M.; Hecht, L. L.; Blank, H.; Schuchmann, H. P.; Gerthsen, D.; Marquardt, C.; Diabaté, S.; Weiss, C.; Marko, D.
2013. Nanotoxicology, 7 (3), 274–293. doi:10.3109/17435390.2011.652207
Khanna, A.; Kauko, O.; Böckelmann, C.; Laine, A.; Schreck, I.; Partanen, J. I.; Szwajda, A.; Bormann, S.; Bilgen, T.; Helenius, M.; Pokharel, Y. R.; Pimanda, J.; Russel, M. R.; Haglund, C.; Cole, K. A.; Kiefström, J.; Aittokallio, T.; Weiss, C.; Ristimäki, A.; Visakorpi, T.; Westermarck, J.
2013. Cancer Research, 73, 6757–6769. doi:10.1158/0008-5472.CAN-13-1002
Kullmann, M. K.; Grubbauer, C.; Goetsch, K.; Jäkel, H.; Podmirseg, S. R.; Trockenbacher, A.; Ploner, C.; Cato, A. C. B.; Weiss, C.; Kofler, R.; Hengst, L.
2013. Cell Cycle, 12, 2625–2635. doi:10.4161/cc.25622
Al-Rawi, M.
2013. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000037535
Dilger, M.; Mülhopt, S.; Schlager, C.; Zimmermann, R.; Orasche, J.; Diabate, S.; Weiss, C.; Krebs, T.; Paur, H. R.
2013. Ultrafine Particles : Sources, Effects, Risks and Mitigation Strategies ; EFCA Internat.Symp., Bruxelles, B, May 16-17, 2013
Dilger, M.; Mülhopt, S.; Schlager, C.; Zimmermann, R.; Orasche, J.; Diabate, S.; Weiss, C.; Krebs, T.; Paur, H. R.
2013. Ultrafine Particles: Sources, Effects, Risks and Mitigation Strategies. EFCA International Symposium, Bruxelles, B, May 16-17, 2013, Karlsruher Institut für Technologie (KIT)
Dilger, M.; Mülhopt, S.; Schlager, C.; Paur, H. R.; Orasche, J.; Zimmermann, R.; Diabate, S.; Weiss, C.
2013. Aerosol Emissions from Fossil Fuel and Biomass Combustion : Chemical Properties, Transformation and Health Effects, Praha, CZ, August 31- September 1, 2013
Zimmermann, R.; Dittmar, T. G.; Kanashova, T.; Buters, J.; Öder, S.; Paur, H.; Schlager, C.; Mülhopt, S.; Dilger, M.; Weiss, C.; Diabate, S.; Harndorf, H.; Stengel, B.; Rabe, R.; Hirvonen, M. R.; Jokiniemi, J.; Torvela, T.; Hiller, K.; Sapccariu, S. C.; BeruBe, K. A.; Wlodarcyzk, A. J.; Sippula, O.; Michalke, B.; Krebs, T.; Kelbg, M.; Tiggesbäumker, J.; Streibel, T.; Karg, E.; Scholtes, S.; Schnelle-Kreis, J.; Lintelmann, J.; Sklorz, M.; Arteaga Salas, M.; Klingbeil, S.; Orasche, J.; Richthammer, P.; Müller, L.; Elsasser, M.; Rheda, A.; Werner, B.; Passig, J.; Gröger, T.; Abbaszade, G.; Radischat, C.
2013. European Aerosol Conference (EAC 2013), Praha, CZ, September 1-6, 2013
Stoehr, L. C.; Diabate, S.; Kielmeier, U.; Mülhopt, S.; Paur, H. R.; Weiss, C.; Oostingh, G. J.; Duschl, A.
2013. 2nd QNano Integrating Conference, Praha, CZ, February 27 - March 1, 2013
Panas, A.; Marquardt, C.; Nalcaci, O.; Bockhorn, H.; Baumann, W.; Paur, H. R.; Mülhopt, S.; Diabate, S.; Weiss, C.
2013. Nanotoxicology, 7 (3), 259–273. doi:10.3109/17435390.2011.652206
Fritsch-Decker, S.; Both, T.; Mülhopt, S.; Paur, H. R.; Weiss, C.; Diabate, S.
2013. Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Halle, 5.-7. März 2013
Khan, A. ul M.; Mikut, R.; Schweitzer, B. ..; Weiss, C.; Reischl, M.
2013. Synergies of Soft Computing and Statistics for Intelligent Data Analysis: Proceedings of the 6th International Conference on Soft Methods in Probability and Statistics, Konstanz, 4.-6. Oktober 2012. Hrsg.: R. Kruse, 459–467, Springer-Verlag. doi:10.1007/978-3-642-33042-1_49
Khan, A.; Reischl, M.; Schweitzer, B.; Weiss, C.; Mikut, R.
2013. Moewes, C. Computational Intelligence in Intelligent Data Analysis Berlin [u.a.] : Springer, 2013 (Studies in Computational Intelligence ; 445), 167–178
Langer, M.; Weiss, C.
2012. doi:10.5445/DIVA/2012-359
Panas, A.; Diabate, S.; Comouth, A.; Saathoff, H.; Paur, H. R.; Mülhopt, S.; Weiss, C.
2012. BWTOX Symposium, Karlsruher Institut für Technologie, 23. November 2012
Al-Rawi, M.; Hug, V.; Diabete, S.; Berret, J. F.; Weiss, C.
2012. BWTOX Symposium, Karlsruher Institut für Technologie, 23. November 2012
Burkhardt, B.; Jung, S. A.; Pfeiffer, E.; Weiss, C.; Metzler, M.
2012. Archives of Toxicology, 86, 643–649. doi:10.1007/s00204-011-0789-8
Schreck, I.; Deigendesch, U.; Burkhardt, B.; Marko, D.; Weiss, C.
2012. 78.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Dresden, 19.-22.März 2012 Naunyn-Schmiedebergs Archives of Pharmacology, 385(2012) Suppl.1, Nr.376 (Abstract)
Al-Rawi, M.; Marquardt, C.; Panas, A.; Ruh, H.; Diabate, S.; Weiss, C.
2012. 78.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Dresden, 19.-22.März 2012 Naunyn-Schmiedebergs Archives of Pharmacology, 385(2012) Suppl.1, Nr.010 (Abstract)
Faust, D.; Schmitt, C.; Oesch, F.; Oesch-Bartlomowicz, B.; Schreck, I.; Weiss, C.; Dietrich, C.
2012. Cell communication and signaling, 10, 6. doi:10.1186/1478-811X-10-6
Eschamps, E.; Weidler, P. G.; Friedrich, F.; Weiss, C.; Diabate, S.
2012. 9th Internat.Symp.on Environmental Geochemistry, Aveiro, P, July 15-21, 2012
Khan, A. M.; Mikut, R.; Schweitzer, B.; Weiss, C.; Reischl, M.
2012. 6th Internat.Conf.on Soft Methods in Probability and Statistics, Konstanz, October 4-6, 2012
Strähle, U.; Scholz, S.; Geisler, R.; Greiner, P.; Hollert, H.; Rastegar, S.; Schumacher, A.; Selderslaghs, I.; Weiss, C.; Witters, H.; Braunbeck, T.
2012. Reproductive toxicology, 33, 128–132. doi:10.1016/j.reprotox.2011.06.121
Schreck, I.; Deigendesch, U.; Burkhardt, B.; Marko, D.; Weiss, C.
2012. Archives of Toxicology, 86, 625–632. doi:10.1007/s00204-011-0781-3
Donauer, J.; Schreck, I.; Liebel, U.; Weiss, C.
2012. Archives of Toxicology, 86 (2), 329–337. doi:10.1007/s00204-011-0757-3
Ruh, H.; Kühl, B.; Brenner-Weiss, G.; Hopf, C.; Diabate, S.; Weiss, C.
2012. Toxicology Letters, 208, 41–50. doi:10.1016/j.toxlet.2011.09.009
Panas, A.
2012. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000026036
Fritsch-Decker, S.; Both, T.; Mülhopt, S.; Paur, H.-R.; Weiss, C.; Diabaté, S.
2011. Particle and fibre toxicology, 8, Art.Nr. 23. doi:10.1186/1743-8977-8-23
Paur, H. R.; Mülhopt, S.; Diabate, S.; Weiss, C.
2011. CEA Nanotox Workshop, Paris, F, December 14-15, 2011
Al-Rawi, M.; Schreck, I.; Diabate, S.; Guber, A.; Strähle, U.; Weiss, C.
2011. Experimentelle und Klinische Pharmakologie und Toxikologie in Baden-Württemberg : 1.Wissenschaftliches Treffen, Schloss Reisensburg, Günzburg, 17.-19.November 2011
Diabate, S.; Bergfeldt, B.; Plaumann, D.; Übel, C.; Weiss, C.
2011. Analytical and Bioanalytical Chemistry, 401, 3197–3212. doi:10.1007/s00216-011-5102-4
Schreck, I.; Al-Rawi, M.; Mingot, J. M.; Scholl, C.; Diefenbacher, M. E.; O’Donnell, P.; Bohmann, D.; Weiss, C.
2011. Biochemical and Biophysical Research Communications, 407, 735–740. doi:10.1016/j.bbrc.2011.03.092
Weiss, C.; Diabate, S.
2011. Archives of Toxicology, 85(2011) Nr.7. doi:10.1007/s00204-011-0707-0
Panas, A.; Diabate, S.; Comouth, A.; Saathoff, H.; Paur, H. R.; Mülhopt, S.; Weiss, C.
2011. Internat.Conf.on Biological Responses to Nanoscale Particles, Essen, September 11-15, 2011
Panas, A.; Comouth, A.; Diabate, S.; Marquardt, C.; Mülhopt, S.; Nalcaci, O.; Paur, H. R.; Saathoff, H.; Bockhorn, H.; Weiss, C.
2011. 2nd German French Workshop on Nanoscience, Landau, August 31 - September 2, 2011
Marquardt, C.
2011. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000024309
Mülhopt, S.; Diabate, S.; Weiss, C.; Paur, H. R.
2011. Neue Entwicklungen bei der Messung und Beurteilung der Luftqualität : Fachtagung, Baden-Baden, 11.-12.Mai 2011 Düsseldorf : VDI-Verl, 2011 (VDI-Berichte ; 2113)
Mülhopt, S.; Diabate, S.; Weiss, C.; Paur, H. R.
2011. Neue Entwicklungen bei der Messung und Beurteilung der Luftqualität : Fachtagung, Baden-Baden, 11.-12.Mai 2011 Düsseldorf : VDI-Verl, 2011 (VDI-Berichte ; 2113), 263–274
Al-Rawi, M.; Diabate, S.; Weiss, C.
2011. Archives of toxicology, 85 (7), 813–826. doi:10.1007/s00204-010-0642-5
Marquardt, C.; Panas, A.; Weiss, C.; Diabate, S.
2011. 77.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Frankfurt, 390.März - 1.April 2011 Naunyn-Schmiedebergs Archives of Pharmacology, 383(2011) Suppl.1, Nr.431 (Abstract)
Al-Rawi, M.; Ali, M.; Diabate, S.; Weiss, C.
2011. 77.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Frankfurt, 390.März - 1.April 2011 Naunyn-Schmiedebergs Archives of Pharmacology, 383(2011) Suppl.1, Nr.430 (Abstract)
Marquardt, C.; Panas, A.; Weiss, C.; Diabate, S.
2011. Naunyn-Schmiedeberg’s archives of pharmacology / Supplement, 77.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Frankfurt, 390.März - 1.April 2011, (Abstract), 383 (1), Nr. 431
Al-Rawi, M.; Ali, M.; Diabate, S.; Weiss, C.
2011. Naunyn-Schmiedeberg’s archives of pharmacology / Supplement 1, 383 (430), 96
Comouth, A.; Paur, H. R.; Baumann, W.; Bockhorn, H.; Diabate, S.; Mülhopt, S.; Nalcaci, O.; Panas, A.; Saathoff, H.; Seifert, H.; Weiss, C.
2010. Internat.Aerosol Conf. (IAC 2010), Helsinki, SF, August 29 - September 3, 2010
Mülhopt, S.; Diabate, S.; Weiss, C.; Seipenbusch, M.; Schlager, C.; Paur, H. R.
2010. Internat.Aerosol Conf. (IAC 2010), Helsinki, SF, August 29 - September 3, 2010 Book of Abstracts
Comouth, A.; Mülhopt, S.; Diabate, S.; Panas, A.; Paur, H. R.; Saathoff, H.; Weiss, C.
2010. Internat.Conf.on Workplace Aerosols, Karlsruhe, June 28 - July 2, 2010
Wahl, M.; Guenther, R.; Yang, L.; Bergman, A.; Straehle, U.; Strack, S.; Weiss, C.
2010. Toxicology Letters, 198, 119–26. doi:10.1016/j.toxlet.2010.06.001
Mülhopt, S.; Comouth, A.; Diabate, S.; Panas, A.; Paur, H. R.; Schlager, C.; Saathoff, H.; Weiss, C.
2010. Internat.Conf.on Workplace Aerosols, Karlsruhe, June 28 - July 2, 2010
Donauer, J.
2010. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000019363
Mülhopt, S.; Diabate, S.; Weiss, C.; Paur, H. R.
2010. 2nd NanoImpactNet Conf., Lausanne, CH, March 9-12, 2010 Book of Abstracts -35 / 139
Marquardt, C.; Diabate, S.; Weiss, C.
2010. Nanotoxicology 2010, Edinburgh, GB, June 2-4, 2010
Ruh, H.; Kühl, B.; Brenner-Weiss, G.; Hopf, C.; Diabate, S.; Weiss, C.
2010. Nanotoxicology 2010, Edinburgh, GB, June 2-4, 2010
Dietrich, C.; Weiss, C.; Bockamp, E.; Brisken, C.; Roskams, T.; Morris, R.; Oesch-Bartlomowicz, B.; Oesch, F.
2010. Archives of Toxicology, 84, 245–51. doi:10.1007/s00204-009-0491-2
Böhmer, F. D.; Weiss, C.; Herrlich, P.
2010. Bradshaw, R.A. [Hrsg.] Handbook of Cell Signaling Vol.3 Amsterdam [u.a.] : Elsevier [u.a.], 2.Ed., 2010, 2225–30
Paur, H.-R.; Baumann, W.; Bockhorn, H.; Comouth, A.; Diabate, S.; Mätzing, H.; Mülhopt, S.; Nalcaci, O. O.; Panas, A.; Ruzin, E.; Saathoff, H.; Seifert, H.; Weiss, C.
2009. European Aerosol Conference Proceedings, 6-11 September 2009, Karlsruhe, Germany. Vol. 1, 18
Weiss, C.
2009. Universität Karlsruhe (TH)
Oesch-Bartlomowicz, B.; Weiss, C.; Dietrich, C.; Oesch, F.
2009. Mutation Research, 680, 83–86. doi:10.1016/j.mrgentox.2009.10.006
Diabate, S.; Weiss, C.; Mülhopt, S.; Paur, H. R.; Niedetzky, V.; Seipenbusch, M.
2009. European Aerosol Conf.(EAC 2009), Karlsruhe, September 6-11, 2009 Abstracts publ.online T013A02
Schreck, I.
2009. Universität Karlsruhe (TH). doi:10.5445/IR/1000015338
Paur, H. R.; Baumann, W.; Bockhorn, H.; Comouth, A.; Diabate, S.; Mätzing, H.; Mülhopt, S.; Nalcaci, O.; Panas, A.; Ruzin, E.; Saathoff, H.; Seifert, H.; Weiss, C.
2009. European Aerosol Conf.(EAC 2009), Karlsruhe, September 6-11, 2009
Mülhopt, S.; Diabate, S.; Krebs, T.; Weiss, C.; Paur, H. R.
2009. 4th Internat.Conf.on Nanotechnology - Occupational and Environmental Health (NanOEH2009), Helsinki, SF, August 26-29, 2009 Book of Abstracts
Mülhopt, S.; Diabate, S.; Krebs, T.; Weiss, C.; Paur, H. R.
2009. Journal of Physics: Conference Series, 170, 012008/1–4. doi:10.1088/1742-6596/170/1/012008
Yang, L.; Ho, N. Y.; Alshut, R.; Legradi, J.; Weiss, C.; Reischl, M.; Mikut, R.; Liebel, U.; Müller, F.; Strähle, U.
2009. Reproductive Toxicology, 28, 245–53. doi:10.1016/j.reprotox.2009.04.013
Mülhopt, S.; Diabate, S.; Grotz, A.; Weiss, C.; Paur, H. R.
2009. Vortr.: Fraunhofer Institut für Toxikologie und Experimentelle Medizin, Hannover, 20.Februar 2008
Schreck, I.; Chudziak, D.; Schneider, S.; Seidel, A.; Platt, K.; Oesch, F.; Weiss, C.
2009. Toxicology, 259, 91–96. doi:10.1016/j.tox.2009.02.006
Comouth, A.; Mülhopt, S.; Saathoff, H.; Rzesanke, D.; Panas, A.; Weiss, C.; Paur, H. R.; Diabate, S.; Leisner, T.
2009. Frühjahrstagung DPG, Fachverband Biologische Physik (2009), Dresden, Germany, March 22–27, 2009
Paur, H. R.; Baumann, W.; Bockhorn, H.; Comouth, A.; Diabate, S.; Mätzing, H.; Mülhopt, S.; Nalcaci, O.; Panas, A.; Ruzin, E.; Saathoff, H.; Seifert, H.; Weiss, C.
2009. Zwischenkolloquium des DFG Schwerpunktprogramms 1313: Biological Responses to Nanoscale Particles, Fulda, 23.-25.Februar 2009
Marquardt, C.; Panas, A.; Weiss, C.; Diabate, S.
2009. 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009 Naunyn-Schmiedebergs Archives of Pharmacology, 379(2009) Suppl.1, Nr.329 (Abstract) In-Vitro Exposure Studies for Toxicity Testing of Engineered Nanoparticles : A Dialogue Between Aerosol Science and Biology, Karlsruhe, September 5-6, 2009
Marquardt, C.; Panas, A.; Weiss, C.; Diabate, S.
2009. Naunyn-Schmiedeberg’s Archives of Pharmacology, 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009, Suppl.1, (Abstract) In-Vitro Exposure Studies for Toxicity Testing of Engineered Nanoparticles : A Dialogue Between Aerosol Science and Biology, Karlsruhe, September 5-6, 2009, 379 (329)
Al-Rawi, M.; Strack, S.; Weiss, C.
2009. 3rd Internat.Symp.on Nanoparticles and the Gastrointestinal Tract, Düsseldorf, 5.-6.März 2009 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009 Naunyn-Schmiedebergs Archives of Pharmacology, 379(2009) Suppl.1, Nr.420 (Abstract)
Schreck, I.; Chudziak, D.; Schneider, S.; Seidel, A.; Platt, K.; Oesch, F.; Weiss, C.
2009. 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009 Naunyn-Schmiedebergs Archives of Pharmacology, 379(2009) Suppl.1, Nr.396 (Abstract)
Al-Rawi, M.; Strack, S.; Weiss, C.
2009. Naunyn-Schmiedeberg’s Archives of Pharmacology, 3rd Internat.Symp.on Nanoparticles and the Gastrointestinal Tract, Düsseldorf, 5.-6.März 2009 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009, Suppl.1, (Abstract), 379 (420)
Schreck, I.; Chudziak, D.; Schneider, S.; Seidel, A.; Platt, K.; Oesch, F.; Weiss, C.
2009. Naunyn-Schmiedeberg’s Archives of Pharmacology, 50.Jahrestagung der Deutschen Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie (DGPT), Mainz, 10.-12.März 2009, Suppl.1, (Abstract), 379 (396)
Mülhopt, S.; Diabate, S.; Krebs, T.; Weiss, C.; Paur, H. R.
2008. Internat.Conf.on Safe Production and Use of Nanomaterials (Nanosafe 2008), Grenoble, F, November 3-7, 2008 Book of Abstracts N⁰O2c-3
Wahl, M.; Guenther, R.; Yang, L.; Straehle, U.; Weiss, C.; Strack, S.
2008. 49th Annual Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie Mainz, March 11-13, 2008 Naunyn-Schmiedeberg’s Archives of Pharmacology, 377(2008) Suppl. (Abstract)
Grico, N.; Donauer, J.; Mattern, D.; Oesch, F.; Weiss, C.
2008. 49th Annual Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie Mainz, March 11-13, 2008 Naunyn-Schmiedeberg’s Archives of Pharmacology, 377(2008) Suppl., (Abstract)
Wahl, M.; Guenther, R.; Yang, L.; Straehle, U.; Weiss, C.; Strack, S.
2008. Naunyn-Schmiedeberg’s Archives of Pharmacology, 49th Annual Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie Mainz, March 11-13, 2008, Suppl. (Abstract), 377, 86
Grico, N.; Donauer, J.; Mattern, D.; Oesch, F.; Weiss, C.
2008. Naunyn-Schmiedeberg’s Archives of Pharmacology, 49th Annual Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie Mainz, March 11-13, 2008, Suppl., (Abstract), 377, 85. doi:10.1007/s00210-008-0275-x
Paur, H. R.; Mülhopt, S.; Weiss, C.; Diabate, S.
2008. Journal für Verbraucherschutz und Lebensmittelsicherheit, 3, 319–29. doi:10.1007/s00003-008-0356-2
Herrlich, P.; Karin, M.; Weiss, C.
2008. Molecular Cell, 29, 279–90. doi:10.1016/j.molcel.2008.01.001
Mülhopt, S.; Diabate, S.; Weiss, C.; Paur, H. R.
2008. European Aerosol Conf., Thessaloniki, GR, August 24-29, 2008
Marquardt, C.; Übel, C.; Ecker, U.; Weiss, C.; Diabate, S.
2008. BW-ToxNet Meeting 2008 Tübingen, 23.Juli 2008
Wahl, M.; Lahni, B.; Guenther, R.; Kuch, B.; Yang, L.; Strähle, U.; Strack, S.; Weiss, C.
2008. Chemosphere, 73, 209–15. doi:10.1016/j.chemosphere.2008.05.025
Diabate, S.; Mülhopt, S.; Paur, H. R.; Weiss, C.
2008. European Aerosol Conf., Thessaloniki, GR, August 24-29, 2008
Diabate, S.; Fritsch, S.; Marquardt, C.; Übel, C.; Weiss, C.
2008. NanoTox 2008 : 2nd Internat.Conf.on Nanotoxicology, Zürich, CH, September 7-10, 2008
Fritsch, S.; Marquardt, C.; Krug, H. F.; Weiss, C.; Diabate, S.
2008. 49.Jahrestagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 11.-13.März 2008 Naunyn-Schmiedebergs Archives of Pharmacology, 377(2008) Suppl.1, (Abstract)
Weiss, C.; Faust, D.; Schreck, I.; Ruff, A.; Farwerck, T.; Melenberg, A.; Schneider, S.; Oesch-Bartlomowicz, B.; Zatloukalova, J.; Vondracek, J.; Oesch, F.; Dietrich, C.
2008. Oncogene, 27, 2198–2207. doi:10.1038/sj.onc.1210859
Paur, H. R.; Bockhorn, H.; Weiss, C.; Saathoff, H.
2007. Begutachtungskolloquium zum DFG Schwerpunktprogramm 1313 ’Bio-Nano-Responses’, Essen, 3.-4.September 2007
Diabate, S.; Weiss, C.
2007. Nachrichten - Forschungszentrum Karlsruhe, 39, 140–44
Strack, S.; Wahl, M.; Weiss, C.; Jay, K.; Kuch, B.; Krug, H. F.
2007. DIOXIN 2006 : 26th Internat.Symp.on Halogenated Persistent Organic Polltants, Oslo, N, August 21-25, 2006 Proc.on CD-ROM (Organohalogen Compounds ; 68)
Strack, S.; Wahl, M.; Guenther, R.; Weiss, C.
2007. 48th Spring Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Mainz, March 13-15, 2007 Naunyn-Schmiedebergs Archives of Pharmacology, 375(2007) Suppl.1. (Abstract)
Faust, D.; Schreck, I.; Oesch, F.; Weiss, C.; Dietrich, C.
2007. 48th Spring Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Mainz, March 13-15, 2007 Naunyn-Schmiedebergs Archives of Pharmacology, 375(2007) (Abstract)
Strack, S.; Wahl, M.; Guenther, R.; Weiss, C.
2007. Naunyn-Schmiedeberg’s Archives of Pharmacology, 48th Spring Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Mainz, March 13-15, 2007, Suppl.1. (Abstract), 375, 101
Faust, D.; Schreck, I.; Oesch, F.; Weiss, C.; Dietrich, C.
2007. Naunyn-Schmiedeberg’s Archives of Pharmacology, 48th Spring Meeting Deutsche Gesellschaft für Experimentelle und Klinische Pharmakologie und Toxikologie, Mainz, March 13-15, 2007, (Abstract), 375, 88
Wahl, M.; Weiss, C.; Kuch, B.; Strack, S.
2007. 4th Internat.Workshop on Brominated Flame Retardants (BFR 2007), Amsterdam, NL, April 24-27, 2007
Diabate, S.; Mülhopt, S.; Paur, H. R.; Weiss, C.; Krug, H. F.
2007. Congress on Alternative Test Methods in Inhalation Toxicology, BfR, Berlin, May 7-9, 2007
Wahl, M.; Günther, R.; Yang, L.; Strähle, U.; Weiss, C.; Strack, S.
2007. 48.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 13.-15.März 2007 Naunyn-Schmiedebergs Archives of Pharmacology, 375(2007) Suppl.1, (Abstract)
Wahl, M.; Günther, R.; Yang, L.; Strähle, U.; Weiss, C.; Strack, S.
2007. Naunyn-Schmiedeberg’s Archives of Pharmacology, 48.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 13.-15.März 2007, Suppl.1, (Abstract), 375, 101
Schneider, S.; Eberhardt, P.; Herzer, U.; Theilacker, N.; Berensmeier, S.; Rogge, T.; Weiß, C.; Cato, A. C. B.
2006. 2nd FRONTIERS Annual Meeting, Acireale, I, October 24-26, 2006
Grico, N.; Oesch, F.; Weiss, C.
2006. 47.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie, Mainz, April 4-6, 2006 Naunyn-Schmiedebergs Archives of Pharmacology, 372(2006) Suppl.7, (Abstract)
Grico, N.; Oesch, F.; Weiss, C.
2006. Naunyn-Schmiedeberg’s Archives of Pharmacology, 47.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie, Mainz, April 4-6, 2006, Suppl.7, (Abstract), 372, 119
Weiss, C.; Faust, D.; Dürk, H.; Kolluri, K. S.; Pelzer, A.; Schneider, S.; Dietrich, C.; Oesch, F.; Göttlicher, M.
2005. Oncogene, 24, 4975–83. doi:10.1038/sj.onc.1208679
Weiss, C.; Kolluri, S. K.; Durk, H.; Pelzer, A.; Oesch, F.; Göttlicher, M.
2004. 45.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 9.-11.März 2004 Naunyn-Schmiedebergs Archives of Pharmacology, 369(2004) Suppl.1., 525 (Abstract)
Weiss, C.; Kolluri, S. K.; Durk, H.; Pelzer, A.; Oesch, F.; Göttlicher, M.
2004. Naunyn-Schmiedeberg’s Archives of Pharmacology, 45.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 9.-11.März 2004, Suppl.1., 525 (Abstract), 369
Göttlicher, M.; Weiss, C.; Balduf, C.; Kolluri, S. K.
2001. 14.Heidelberger Cytometrie-Symp., Heidelberg, 18.-20.Oktober 2001
Göttlicher, M.; Kolluri, K. S.; Balduf, C.; Weiss, C.
2000. 6th Internat.Dahlem Symp.on Cellular Signal Recognition and Transduction and Fall Meeting of the German Society of Experimental and Clinical Pharmacology and Toxicology, Berlin, September 27-30, 2000
Göttlicher, M.; Kolluri, S. K.; Balduf, C.; Weiss, C.
2000. 8th KBF Symp.Complexity in Medicine, Köln, September 13-15, 2000
Weiss, C.
1999. Karlsruhe 1999. (Wissenschaftliche Berichte. FZKA. 6381.) Fak. f. Bio- und Geowissenschaften, Diss. v. 5.5.1999., Universität Karlsruhe (TH)
Kolluri, S. K.; Weiss, C.; Koff, A.; Göttlicher, M.
1999. Genes and Development, 13, 1742–53
Weiss, C.
1999. Wissenschaftliche Berichte, FZKA-6381 (Oktober 99). doi:10.5445/IR/270046438
Weiss, C.; Kolluri, S. K.; Koff, A.; Göttlicher, M.
1999. The Salk Institute for Biological Studies - Oncogene and Growth Control Meeting, La Jolla, Calif., August 18-22, 1999
Göttlicher, M.; Pelzer, A.; Litfin, M.; Kolluri, S. K.; Weiss, C.
1999. DIOXIN 99 : 19th Internat.Symp.on Halogenated Environmental Organic Pollutants and Persistent Organic Pollutants (POPs), Venezia, I, September 12-17, 1999
Göttlicher, M.; Kolluri, S. K.; Weiss, C.
1998. International Blaubeuren Symposium, Blaubeuren, August 23-25, 1998
Kolluri, S. K.; Weiss, C.; Göttlicher, M.
1998. 39.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 17.-19.März 1998 Keystone Symp.on Molecular and Cellular Biology: The Cell Cycle, Keystone, Colo., March 27 - April 2, 1998
Kolluri, K.; Weiss, C.; Göttlicher, M.
1997. 38.Fruehjahrstagung der Deutschen Gesellschaft für Pharmakologie und Toxikologie, Mainz, 11.-13.März 1997
Göttlicher, M.; Kolluri, K.; Weiss, C.
1997. Nachrichten - Forschungszentrum Karlsruhe, 29, 211–20
Weiss, C.; Kolluri, K.; Kiefer, F.; Göttlicher, M.
1996. 37.Frühjahrstagung der Deutschen Gesellschaft für Pharmakologie und Toxikologie, Mainz, 12.-14.Mäerz 1996
Weiss, C.; Kolluri, S. K.; Kiefer, F.; Göttlicher, M.
1996. Experimental Cell Research, 226, 154–63
Weiss, C.; Kiefer, F.; Göttlicher, M.
1995. 36.Frühjahrstagung der Deutschen Gesellschaft für experimentelle klinische Pharmakologie und Toxikologie, Mainz, 14.-16.Mäerz 1995
Weiss, C.
1995. Diplomarbeit, Universtität Karlsruhe 1995
Read More
Best paper award ESN
Our collaborative work involving the screening centre and three IBCS-BIP groups (Dickmeis-, Rastegar-, Weiss-lab) received the Environmental Science: Nano best paper award 2022! Within the EU FP7 project NanoMILE, we joined forces with scientists from different disciplines to study the impact of specifically synthesized and well characterized nanomaterials on a variety of cell lines and zebrafish embryos. Ten years after the project was launched, we finally published our detailed analysis on the importance of surface properties of nanomaterials for detrimental effects on zebrafish development. Such highly interdisciplinary work across countries is indeed required to support the safe development of nanomaterials and truly inspiring, but also challenging, for all the researchers involved at different career stages ranging from bachelor students, PhD and post-doctoral students up to senior scientists (https://pubs.rsc.org/en/content/articlelanding/2023/en/d3en90021e).
Award for the best presentation at the Annual Consortium Meeting of the European Project Precision Toxicology
At the Annual Consortium Meeting of the European Project Precision Toxicology held in Barcelona in June 2022, Dr. Gaëlle Hayot (Dickmeis lab) and PhD Student Christina Cramer von Clausbruch (Weiss lab) won not only two nice coffee mugs but also the award for the best presentation showcasing their work on zebrafish and human cells as models to improve chemical safety. Congratulations!

Public Outreach
KIT researchers Achim Dittler (MVM), Sonja Mülhopt (ITC) and Carsten Weiss (IBCS-BIP) discuss their latest findings concerning health effects of wood smoke as main constituent of air pollution on German TV (https://www.3sat.de/wissen/nano/210331-holzofen-nano-102.html).
Precision Toxicology consortium aims to protect human health from effects of harmful chemicals
A major research project to shape regulation and policy on chemical safety without the use of animal testing has been launched with the aid of €19.3M funding from the European Commission.
Interview about the project in Wiley Analytical Science: https://analyticalscience.wiley.com/content/news-do/auswirkungen-von-chemikalien-auf-die-menschliche-gesundheit
