Extracellular Matrix Biology
ABOUT THE LAB
The central aim of the Sleeman lab is to understand the process of metastasis and to use this knowledge to develop novel anti-cancer therapies. Working mainly on breast cancer, lung cancer and melanoma, our research currently includes studies on genetic changes and signaling pathways that promote metastasis, tumor-induced lymphangiogenesis, dissemination via the lymphatics and therapy resistance. Another important focus is to understand the microenvironmental regulation of metastatic spread and dormancy, including how changes in the extracellular matrix composition, the induction of senescence and pro-inflammatory signaling molecules act as regulators of dissemination and metastatic outgrowth.
In additional to being Head of Department at IBCS-BIP, Jonathan Sleeman is Professor of Microvascular Biology and Pathobiology at the University of Heidelberg, Medical Faculty Mannheim, Germany, where he is a core member of the European Center for Angiocience (ECAS). Our research is carried out at both locations. More information about our activities can be found using the link below.
RESEARCH AT IBCS-BIP
The extracellular matrix (ECM) that surrounds cells is more than just an inert scaffold. It is a rich source of microenvironmental signals and information, and plays a major role in regulating cell behaviour through the wide range of chemical and biophysical cues it provides. Dynamic changes in the synthesis, modification and degradation of the ECM provides a further level of regulatory complexity. Our work at IBCS-BIP focuses on understanding how cells respond to and regulate the ECM microenvironment, and how this two-way flow of biological information goes awry in pathological contexts.
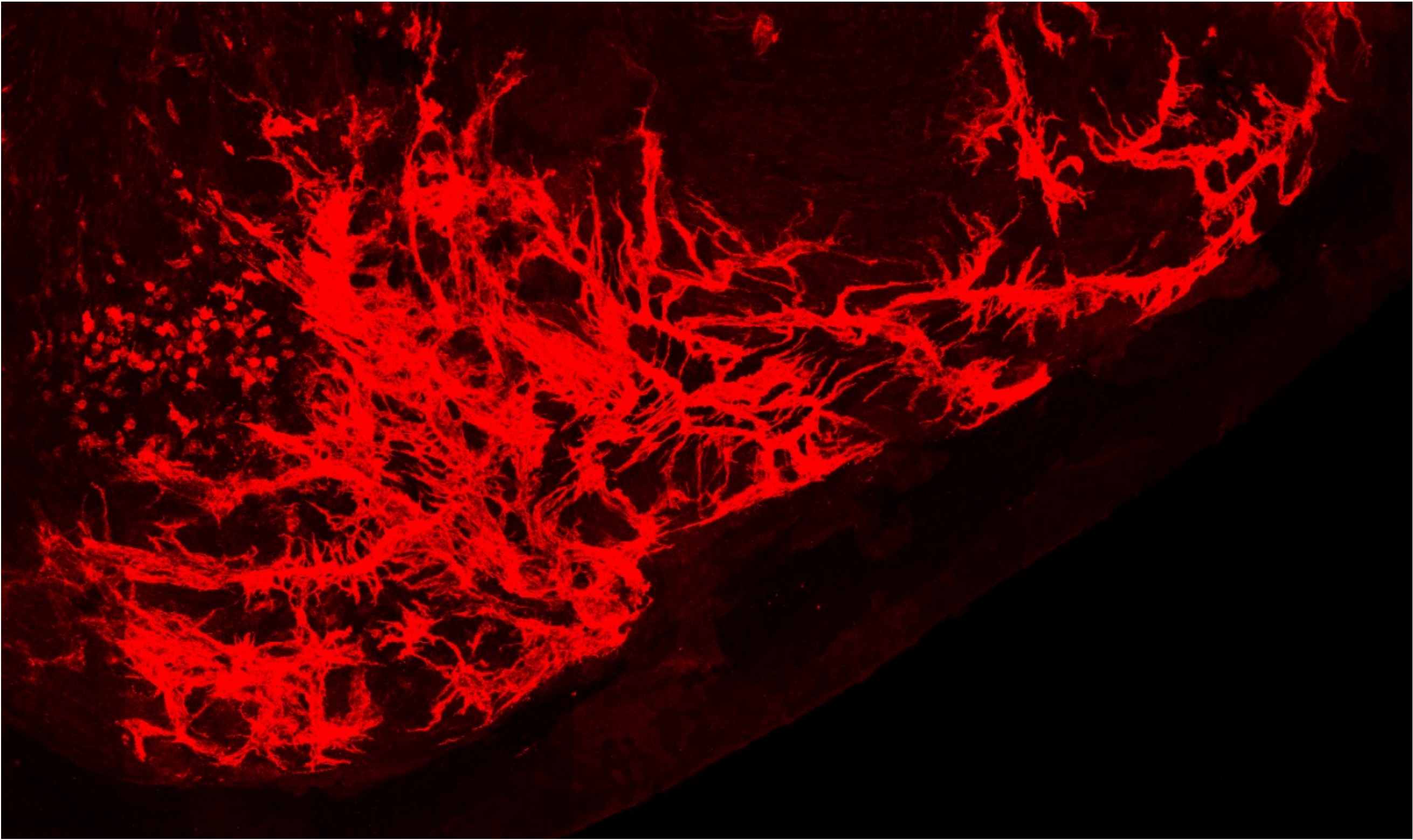
Focusing on the regulation of malignant progression, we are investigating how the ECM regulates cancer stem cell properties, and can thereby promote metastasis. For example, we have discovered matrix-assisted autocrine signalling as a means through which particular ECM environments dramatically reduce the diffusion of signalling molecules produced by cancer cells, and thereby foster autocrine stimulation of stemness properties[1]. These properties are dependent on autocrine signal-induced expression of the transcriptional regulators Id1 and Id3. Together with colleagues at IBCS-FMS, we have identified and patented[2] a novel class of coumarins that inhibit expression of the Id1 and Id3 proteins. Currently we are exploring the potential clinical utility of these compounds for diseases such as cancer.

B. Endogenously-produced BMP accumulates pericellularly around melanoma cells grown in 3D matrix
From Sedlmeier et al., Adv Therapeutics, 2021
Another major focus of our work concerns the ECM glycosaminoglycan hyaluronic acid (HA), which is a major regulator of the viscoelastic and hydration properties of the ECM, and which can thereby impact on cell behaviour directly, or by engaging with cell surface receptors such as CD44. Accordingly, the balance between HA synthesis and degradation is tightly regulated homeostatically, and perturbed HA metabolism contributes to many disease processes, including cancer. Increased expression of CEMIP, a secreted protein that can degrade HA, is associated with numerous diseases such as cancer, arthritis, multiple sclerosis and inflammatory bowel disease[3]. The identification of inhibitors that suppress the HA degrading activity of CEMIP therefore has the potential to find clinical application for the treatment of these diseases. We have previously identified highly sulfated hyaluronic acid[4], and more recently other sulfated polymeric hydrocarbons such as heparin and dextran sulfate as being potent inhibitors of the CEMIP hyaluronidase activity[5]. Current work focuses on understanding the role of CEMIP in resistance to targeted therapy in melanoma, and the possible clinical application of CEMIP inhibitors in diseases such as cancer and arthritis.
[1] Sedlmeier G, Al‐Rawi V, Buchert J, Yserentant K, Rothley M, Steshina A, Gräßle S, Wu R‐L, Hurrle T, Richer W, Decraene C, Thiele W, Utikal J, Abuillan W, Tanaka M, Herten DP, Hill CS, Garvalov BK, Jung N, Bräse S and Sleeman JP (2021). Id1 and Id3 Are Regulated Through Matrix‐Assisted Autocrine BMP Signaling and Represent Therapeutic Targets in Melanoma. Adv. Therap. 2000065. DOI:10.1002/adtp.202000065
[2] EP 3 760 614 B1 CHEMICAL INHIBITORS OF ID PROTEINS FOR THE TREATMENT OF CANCER AND OTHER DISEASES
[3] Domanegg K, Sleeman JP, Schmaus A. CEMIP, a Promising Biomarker That Promotes the Progression and Metastasis of Colorectal and Other Types of Cancer. Cancers (Basel). 2022 Oct 18;14(20):5093.
[4] Schmaus A, Rothley M, Schreiber C, Möller S, Roßwag S, Franz S, Garvalov BK, Thiele W, Spataro S, Herskind C, Prunotto M, Anderegg U, Schnabelrauch M, Sleeman J. Sulfated hyaluronic acid inhibits the hyaluronidase CEMIP and regulates the HA metabolism, proliferation and differentiation of fibroblasts. Matrix Biol. 2022 May;109:173-191.
[5] Schmaus A, Spataro S, Sallmann P, Möller S, Scapozza L, Prunotto M, Sleeman JP. A Novel, Cell-Compatible Hyaluronidase Activity Assay Identifies Dextran Sulfates and Other Sulfated Polymeric Hydrocarbons as Potent Inhibitors for CEMIP. Cells. 2025 Jan 11;14(2):101. doi: 10.3390/cells14020101.
Prof. Dr. Jonathan Sleeman
- Head of Department
- Group: Sleeman
- Room: 102
CN 0304 - Phone: +49 721 608-26089
- jonathan sleeman2 ∂does-not-exist.kit edu
- MS Teams-Chat
Karlsruhe Institute of Technology (KIT)
Campus North
Institute of Biological and Chemical Systems (IBCS)
Building 319
Hermann-von-Helmholtz-Platz 1
76344 Eggenstein-Leopoldshafen
Germany
Publications
Wondimu, S. F.; Khanduri, R.; Atanga, J.; Hippler, M.; Hofmann, A. F.; Hussal, C.; Kohler, D.; Krämmer, S.; Bog, U.; Wienhold, T.; Koenig, M.; Köber, S.; Mappes, T.; Lahann, J.; Kalt, H.; Freude, W.; Sleeman, J.; Warnecke, A.; Erbes, T.; Juhasz-Böss, I.; Koos, C. G.; Nazarenko, I.
2026. Lab on a Chip, 26 (2), 375–390. doi:10.1039/D5LC00269A
Mayer, S. A.; Thomas, B.; Heuer, M.; Brune, J. C.; Eras, V.; Schuster, K.; Knoedler, L.; Schaefer, R. L.; Thiele, W.; Sleeman, J. P.; Dimmler, A.; Heimel, P.; Kneser, U.; Bigdeli, A. K.; Falkner, F.
2025. Tissue Engineering - Part A, 31 (5-6), 234–243. doi:10.1089/ten.tea.2024.0037
Falkner, F.; Mayer, S. A.; Thomas, B.; Dimmler, A.; Heimel, P.; Schneider, K.; Kengelbach-Weigand, A.; Kramer, A.-M.; Schaefer, R. L.; Panayi, A. C.; Sleeman, J. P.; Thiele, W.; Podesser, B.; Bergmeister, H.; Kneser, U.; Schmidt, V. J.; Bigdeli, A. K.
2025. Tissue Engineering Part A. doi:10.1177/19373341251372950
Hildenbrand, N.; Thomas, B.; Falkner, F.; Schulte, M.; Mueller, S.; Jugold, M.; Garvalov, B. K.; Dimmler, A.; Schmidt, V. J.; Sleeman, J. P.; Kneser, U.; Thiele, W.
2025. Regenerative Engineering and Translational Medicine. doi:10.1007/s40883-025-00464-3
Schmaus, A.; Spataro, S.; Sallmann, P.; Möller, S.; Scapozza, L.; Prunotto, M.; Sleeman, J. P.
2025. Cells, 14 (2), Art.-Nr.: 101. doi:10.3390/cells14020101
Deo, A.; Sleeman, J. P.; Shaked, Y.
2024. Clinical and Experimental Metastasis, 41 (4), 495–507. doi:10.1007/s10585-023-10243-5
Sleeman, J. P.; Haier, J.
2024. Clinical & Experimental Metastasis, 41 (3), 159–161. doi:10.1007/s10585-024-10281-7
Falkner, F.; Mayer, S. A.; Heuer, M.; Brune, J.; Helt, H.; Bigdeli, A. K.; Dimmler, A.; Heimel, P.; Thiele, W.; Sleeman, J. P.; Bergmeister, H.; Schneider, K. H.; Kneser, U.; Thomas, B.
2024. Plastic and Reconstructive Surgery, 153 (1), 130 – 141. doi:10.1097/PRS.0000000000010511
Haier, J.; Sleeman, J.
2023. Clinical & Experimental Metastasis, 40 (6), 443–444. doi:10.1007/s10585-023-10239-1
Spataro, S.; Guerra, C.; Cavalli, A.; Sgrignani, J.; Sleeman, J.; Poulain, L.; Boland, A.; Scapozza, L.; Moll, S.; Prunotto, M.
2023. The FEBS Journal, 290 (16), 3946–3962. doi:10.1111/febs.16600
Thomas, B.; Warszawski, J.; Falkner, F.; Bleichert, S.; Haug, V.; Bigdeli, A. K.; Schulte, M.; Hoffmann, S. H. L.; Garvalov, B. K.; Schreiber, C.; Takamiya, M.; Sleeman, J. P.; Schmidt, V. J.; Kneser, U.; Pichler, B. J.; Dimmler, A.; Thiele, W.
2023. Plastic and reconstructive surgery, 152 (1), 96e – 109e. doi:10.1097/PRS.0000000000010146
Sleeman, J. P.
2023. Clinical & Experimental Metastasis, 40 (2), 123–124. doi:10.1007/s10585-023-10205-x
Albrecht, M.; Sticht, C.; Wagner, T.; Hettler, S. A.; De La Torre, C.; Qiu, J.; Gretz, N.; Albrecht, T.; Yard, B.; Sleeman, J. P.; Garvalov, B. K.
2023. Scientific Reports, 13, Article no: 17985. doi:10.1038/s41598-023-45139-7
Grube, M.; Dimmler, A.; Schmaus, A.; Saup, R.; Wagner, T.; Garvalov, B. K.; Sleeman, J. P.; Thiele, W.
2023. Clinical and Experimental Metastasis. doi:10.1007/s10585-023-10249-z
Saup, R.; Nair, N.; Shen, J.; Schmaus, A.; Thiele, W.; Garvalov, B. K.; Sleeman, J. P.
2023. Biomedicines, 11 (4), Art.-Nr.: 1038. doi:10.3390/biomedicines11041038
Cotarelo, C. L.; Schad, A.; Schmidt, M.; Hönig, A.; Sleeman, J. P.; Thaler, S.
2023. Cancers, 15 (6), Art.-Nr.: 1860. doi:10.3390/cancers15061860
Falkner, F.; Mayer, S. A.; Thomas, B.; Zimmermann, S. O.; Walter, S.; Heimel, P.; Thiele, W.; Sleeman, J. P.; Bigdeli, A. K.; Kiss, H.; Podesser, B. K.; Kneser, U.; Bergmeister, H.; Schneider, K. H.
2023. Bioengineering, 10 (3), Artkl.Nr.: 337. doi:10.3390/bioengineering10030337
Schmaus, A.; Rothley, M.; Schreiber, C.; Möller, S.; Roßwag, S.; Franz, S.; Garvalov, B. K.; Thiele, W.; Spataro, S.; Herskind, C.; Prunotto, M.; Anderegg, U.; Schnabelrauch, M.; Sleeman, J.
2022. Matrix Biology, 109, 173–191. doi:10.1016/j.matbio.2022.04.001
Domanegg, K.; Sleeman, J. P.; Schmaus, A.
2022. Cancers, 14 (20), Art.Nr. 5093. doi:10.3390/cancers14205093
Schreiber, C.; Gruber, A.; Roßwag, S.; Saraswati, S.; Harkins, S.; Thiele, W.; Foroushani, Z. H.; Munding, N.; Schmaus, A.; Rothley, M.; Dimmler, A.; Tanaka, M.; Garvalov, B. K.; Sleeman, J. P.
2022. Cancer Letters, 533, Art.-Nr.: 215600. doi:10.1016/j.canlet.2022.215600
Roßwag, S.; Sleeman, J. P.; Thaler, S.
2021. Cells, 10 (11), Art. Nr.: 2868. doi:10.3390/cells10112868
Hippe, A.; Braun, S. A.; Oláh, P.; Gerber, P. A.; Schorr, A.; Seeliger, S.; Holtz, S.; Jannasch, K.; Pivarcsi, A.; Buhren, B.; Schrumpf, H.; Kislat, A.; Bünemann, E.; Steinhoff, M.; Fischer, J.; Lira, S. A.; Boukamp, P.; Hevezi, P.; Stoecklein, N. H.; Hoffmann, T.; Alves, F.; Sleeman, J.; Bauer, T.; Klufa, J.; Amberg, N.; Sibilia, M.; Zlotnik, A.; Müller-Homey, A.; Homey, B.
2021. British journal of cancer, 125 (9), 1318. doi:10.1038/s41416-021-01563-y
Haier, J.; Sleeman, J.; Schäfers, J.
2021. Clinical & experimental metastasis, 38, Art.Nr. 429. doi:10.1007/s10585-021-10107-w
Sedlmeier, G.; Al-Rawi, V.; Buchert, J.; Yserentant, K.; Rothley, M.; Steshina, A.; Gräßle, S.; Wu, R.-L.; Hurrle, T.; Richer, W.; Decraene, C.; Thiele, W.; Utikal, J.; Abuillan, W.; Tanaka, M.; Herten, D.-P.; Hill, C. S.; Garvalov, B. K.; Jung, N.; Bräse, S.; Sleeman, J. P.
2021. Advanced therapeutics, 4 (2), Art.-Nr.: 2000065. doi:10.1002/adtp.202000065
Leong, S. P.; Witz, I. P.; Sagi-Assif, O.; Izraely, S.; Sleeman, J.; Piening, B.; Fox, B. A.; Bifulco, C. B.; Martini, R.; Newman, L.; Davis, M.; Sanders, L. M.; Haussler, D.; Vaske, O. M.; Witte, M.
2021. Clinical & experimental metastasis, 39, 85–99. doi:10.1007/s10585-021-10097-9
Kyjacova, L.; Saup, R.; Rothley, M.; Schmaus, A.; Wagner, T.; Boßerhoff, A.; Garvalov, B. K.; Thiele, W.; Sleeman, J. P.
2021. Journal of Clinical Medicine, 10 (22), Art.Nr. 5459. doi:10.3390/jcm10225459
Kyjacova, L.; Saup, R.; Rönsch, K.; Wallbaum, S.; Dukowic-Schulze, S.; Foss, A.; Scherer, S. D.; Rothley, M.; Neeb, A.; Grau, N.; Thiele, W.; Thaler, S.; Cremers, N.; Sticht, C.; Gretz, N.; Garvalov, B. K.; Utikal, J.; Sleeman, J. P.
2021. Oncogene, 40, 6494–6412. doi:10.1038/s41388-021-02027-6
Roßwag, S.; Cotarelo, C. L.; Pantel, K.; Riethdorf, S.; Sleeman, J. P.; Schmidt, M.; Thaler, S.
2021. Cancers, 13 (8), Art.-Nr.: 1810. doi:10.3390/cancers13081810
Haier, J.; Sleeman, J.; Schäfers, J.
2020. Clinical & experimental metastasis, 37 (5), 561–563. doi:10.1007/s10585-020-10050-2
Hippe, A.; Braun, S. A.; Oláh, P.; Gerber, P. A.; Schorr, A.; Seeliger, S.; Holtz, S.; Jannasch, K.; Pivarcsi, A.; Buhren, B.; Schrumpf, H.; Kislat, A.; Bünemann, E.; Steinhoff, M.; Fischer, J.; Lira, S. A.; Boukamp, P.; Hevezi, P.; Stoecklein, N. H.; Hoffmann, T.; Alves, F.; Sleeman, J.; Bauer, T.; Klufa, J.; Amberg, N.; Sibilia, M.; Zlotnik, A.; Müller-Homey, A.; Homey, B.
2020. British journal of cancer, 123 (6), 942–954. doi:10.1038/s41416-020-0943-2
Haier, J.; Sleeman, J.; Schäfers, J.
2020. Clinical & experimental metastasis, 37 (4), 447–450. doi:10.1007/s10585-020-10046-y
Thiele, W.; Kyjacova, L.; Köhler, A.; Sleeman, J. P.
2020. Laboratory animals, 54 (4), 391–396. doi:10.1177/0023677219873684
Foss, A.; Muñoz-Sagredo, L.; Sleeman, J.; Thiele, W.
2020. Clinical & experimental metastasis, 37 (1), 47–67. doi:10.1007/s10585-019-10009-y
Funck, F.; Pahl, J.; Kyjacova, L.; Freund, L.; Oehrl, S.; Gräbe, G.; Pezer, S.; Hassel, J. C.; Sleeman, J.; Cerwenka, A.; Schäkel, K.
2020. OncoImmunology, 9 (1), Article-Nr.: 1808424. doi:10.1080/2162402X.2020.1808424
Sun, Q.; Picascia, T.; Khan, A. ul M.; Brenna, C.; Heuveline, V.; Schmaus, A.; Sleeman, J. P.; Gretz, N.
2020. Experimental lung research, 46 (10), 393–408. doi:10.1080/01902148.2020.1829183
Roßwag, S.; Thiede, G.; Sleeman, J. P.; Thaler, S.
2020. Cancers, 12 (9), Art. Nr.: 2689. doi:10.3390/cancers12092689
Nwosu, Z. C.; Piorońska, W.; Battello, N.; Zimmer, A. D.; Dewidar, B.; Han, M.; Pereira, S.; Blagojevic, B.; Castven, D.; Charlestin, V.; Holenya, P.; Lochead, J.; De La Torre, C.; Gretz, N.; Sajjakulnukit, P.; Zhang, L.; Ward, M. H.; Marquardt, J. U.; Magliano, M. P. di; Lyssiotis, C. A.; Sleeman, J.; Wölfl, S.; Ebert, M. P.; Meyer, C.; Hofmann, U.; Dooley, S.
2020. EBioMedicine, 54, Art.Nr.: 102699. doi:10.1016/j.ebiom.2020.102699
Haier, J.; Sleeman, J.; Schäfers, J.
2020. Clinical & experimental metastasis, 37 (1), 1–5. doi:10.1007/s10585-019-10014-1
Haier, J.; Sleeman, J.; Schäfers, J.
2019. Clinical & experimental metastasis, 36 (6), 477–480. doi:10.1007/s10585-019-10003-4
Sleeman, J. P.
2019. Clinical & experimental metastasis, 36 (2), 69. doi:10.1007/s10585-019-09962-5
Grau, N.; Scherer, S. D.; Rothley, M.; Roßwag, S.; Thiele, W.; Stoecklein, N. H.; Cremers, N.; Thaler, S.; Garvalov, B. K.; Sleeman, J. P.
2019. International journal of cancer, 147 (4), 1190–1198. doi:10.1002/ijc.32766
Schreiber, C.; Saraswati, S.; Harkins, S.; Gruber, A.; Cremers, N.; Thiele, W.; Rothley, M.; Plaumann, D.; Korn, C.; Armant, O.; Augustin, H. G.; Sleeman, J. P.
2019. (G. S. Barsh, Ed.) PLoS Genetics, 15 (6), Art.Nr.: e1008216. doi:10.1371/journal.pgen.1008216
Voglstaetter, M.; Thomsen, A. R.; Nouvel, J.; Koch, A.; Jank, P.; Navarro, E. G.; Gainey-Schleicher, T.; Khanduri, R.; Groß, A.; Rossner, F.; Blaue, C.; Franz, C. M.; Veil, M.; Puetz, G.; Hippe, A.; Dindorf, J.; Kashef, J.; Thiele, W.; Homey, B.; Greco, C.; Boucheix, C.; Baur, A.; Erbes, T.; Waller, C. F.; Follo, M.; Hossein, G.; Sers, C.; Sleeman, J.; Nazarenko, I.
2019. The journal of pathology, 248 (4), 421–437. doi:10.1002/path.5281
Wu, R.-L.; Sedlmeier, G.; Kyjacova, L.; Schmaus, A.; Philipp, J.; Thiele, W.; Garvalov, B. K.; Sleeman, J. P.
2018. Scientific reports, 8 (1), Article no 14913. doi:10.1038/s41598-018-33337-7
Sleeman, J. P.
2018. Clinical & experimental metastasis, 35 (8), 713–714. doi:10.1007/s10585-018-9948-0
Nwosu, Z. C.; Battello, N.; Rothley, M.; Piorońska, W.; Sitek, B.; Ebert, M. P.; Hofmann, U.; Sleeman, J.; Wölfl, S.; Meyer, C.; Megger, D. A.; Dooley, S.
2018. Journal of experimental & clinical cancer research, 37 (1), Article no 267. doi:10.1186/s13046-018-0939-4
Araos, J.; Sleeman, J. P.; Garvalov, B. K.
2018. Clinical & experimental metastasis, 35 (7), 563–599. doi:10.1007/s10585-018-9930-x
Bauer, J.; Rothley, M.; Schmaus, A.; Quagliata, L.; Ehret, M.; Biskup, M.; Orian-Rousseau, V.; Jackson, D. G.; Pettis, R. J.; Harvey, A.; Bräse, S.; Thiele, W.; Sleeman, J. P.
2018. Journal of molecular medicine, 96 (2), 199–209. doi:10.1007/s00109-017-1615-4
Antoni, C. H.; McDuffie, Y.; Bauer, J.; Sleeman, J. P.; Boehm, H.
2018. Frontiers in Bioengineering and Biotechnology, 6. doi:10.3389/fbioe.2018.00025
Nwosu, Z. C.; Battello, N.; Rothley, M.; Piorońska, W.; Sitek, B.; Ebert, M. P.; Hofmann, U.; Sleeman, J.; Wölfl, S.; Meyer, C.; Megger, D. A.; Dooley, S.
2018. Journal of experimental & clinical cancer research, 37 (1), Art. Nr.: 211. doi:10.1186/s13046-018-0872-6
Thiele, W.; Rothley, M.; Dimmler, A.; Bugert, P.; Salomó Coll, C.; Sleeman, J. P.
2018. Clinical & experimental metastasis, 35 (7), 679–689. doi:10.1007/s10585-018-9924-8
Haier, J.; Sleeman, J. P.
2018. Clinical & experimental metastasis, 35, 1–2. doi:10.1007/s10585-018-9880-3
Veselkov, K.; Sleeman, J.; Claude, E.; Vissers, J. P. C.; Galea, D.; Mroz, A.; Laponogov, I.; Towers, M.; Tonge, R.; Mirnezami, R.; Takats, Z.; Nicholson, J. K.; Langridge, J. I.
2018. Scientific reports, 8 (1), Art.Nr.: 4053. doi:10.1038/s41598-018-22499-z
Haier, J.; Sleeman, J. P.
2017. Clinical & experimental metastasis, 34 (8), 441–442. doi:10.1007/s10585-018-9879-9
Thaler, S.; Schmidt, M.; Roßwag, S.; Thiede, G.; Schad, A.; Sleeman, J. P.
2017. OncoTarget, 8 (42), 72281–72301. doi:10.18632/oncotarget.20261
Sleeman, J. P.
2017. Clinical & experimental metastasis, 1–3. doi:10.1007/s10585-017-9851-0
Sleeman, J. P.
2017. Clinical & experimental metastasis, 34 (3-4), 197–198. doi:10.1007/s10585-017-9845-y
Thaler, S.; Schmidt, M.; Thiede, G.; Schad, A.; Sleeman, J. P.
2017. Cancer research, 77 (4 Supplement), P6–12. doi:10.1158/1538-7445.SABCS16-P6-12-14
Sedlmeier, G.; Sleeman, J. P.
2017. Biochemical Society transactions, 45 (1), 173–181. doi:10.1042/BST20160263
Stott, S. R. W.; Hayat, S.; Carnwath, T.; Garas, S.; Sleeman, J. P.; Barker, R. A.
2017. PLoS one, 12 (2), e0171748. doi:10.1371/journal.pone.0171748
Thaler, S.; Schmidt, M.; Thiede, G.; Schad, A.; Sleeman, J. P.
2016. San Antonio Breast Cancer Symposium, San Antonio, TX, December 6-10, 2016
Hanke-Roos, M.; Fuchs, K.; Maleschlijski, S.; Sleeman, J.; Orian-Rousseau, V.; Rosenhahn, A.
2016. Cell adhesion & migration, 11 (5-6), 476–487. doi:10.1080/19336918.2016.1260809
Abu-Tayeh, H.; Weidenfeld, K.; Zhilin-Roth, A.; Schif-Zuck, S.; Thaler, S.; Cotarelo, C.; Tan, T. Z.; Thiery, J. P.; Green, J. E.; Klorin, G.; Sabo, E.; Sleeman, J. P.; Tzukerman, M.; Barkan, D.
2016. Cell death & disease, 7 (12), Art. Nr.: e2491. doi:10.1038/cddis.2016.387
Cotarelo, C. L.; Schad, A.; Kirkpatrick, C. J.; Sleeman, J. P.; Springer, E.; Schmidt, M.; Thaler, S.
2016. OncoTarget, 7 (46), 74846–74859. doi:10.18632/oncotarget.12432
Alishekevitz, D.; Gingis-Velitski, S.; Kaidar-Person, O.; Gutter-Kapon, L.; Scherer, S. D.; Raviv, Z.; Merquiol, E.; Ben-Nun, Y.; Miller, V.; Rachman-Tzemah, C.; Timaner, M.; Mumblat, Y.; Ilan, N.; Loven, D.; Hershkovitz, D.; Satchi-Fainaro, R.; Blum, G.; P. Sleeman, J.; Vlodavsky, I.; Shaked, Y.
2016. Cell reports, 17 (5), 1344–1356. doi:10.1016/j.celrep.2016.09.083
Li, J.; Kwiatkowska, B.; Lu, H.; Voglstätter, M.; Ueda, E.; Grunze, M.; Sleeman, J.; Levkin, P. A.; Nazarenko, I.
2016. ACS applied materials & interfaces, 8 (42), 28554–28565. doi:10.1021/acsami.6b11338
Sleeman, J. P.
2016. Clinical & experimental metastasis, 33, 741–742. doi:10.1007/s10585-016-9823-9
Scherer, S. D.; Bauer, J.; Schmaus, A.; Neumaier, C.; Herskind, C.; Veldwijk, M. R.; Wenz, F.; Sleeman, J. P.
2016. PLoS one, 11 (9), Art. Nr.: e0162221. doi:10.1371/journal.pone.0162221
Cremers, N.; Neeb, A.; Uhle, T.; Dimmler, A.; Rothley, M.; Allgayer, H.; Fodde, R.; Sleeman, J. P.; Thiele, W.
2016. PLoS one, 11 (3), e0151468. doi:10.1371/journal.pone.0151468
Seidl, C.; Ungelenk, J.; Zittel, E.; Bergfeldt, T.; Sleeman, J. P.; Schepers, U.; Feldmann, C.
2016. ACS nano, 10 (3), 3149–3157. doi:10.1021/acsnano.5b03060
Krachulec, J. M.; Sedlmeier, G.; Thiele, W.; Sleeman, J. P.
2016. Biochemistry and Cell Biology, 94 (3), 289–296. doi:10.1139/bcb-2015-0150
Sleeman, J. P.
2015. Journal of molecular medicine, 93, 1173–1184. doi:10.1007/s00109-015-1351-6
Sleeman, J. P.
2015. Journal of molecular medicine, 93, 1171–1172. doi:10.1007/s00109-015-1361-4
Tahler, S.; Theide, G.; Hengstler, J. G.; Schad, A.; Schmidt, M.; Sleeman, J. P.
2015. International journal of cancer, 137, 686–697. doi:10.1002/ijc.29404
Klusmeier, S.; Quagliata, L.; Sleeman, J. P.
2015. 15th International Biennial Congress of the Metastasis Research Society, Heidelberg, June 28 - July 1, 2014 Clinical and Experimental Metastasis, 32(2015) (Abstract)
Jerabek, L.; Schmaus, A.; Sleeman, J. P.
2015. 15th International Biennial Congress of the Metastasis Research Society, Heidelberg, June 28 - July 1, 2014 Clinical and Experimental Metastasis, 32(2015) (Abstract)
Rönsch, K.; Wallbaum, S.; Neeb, A.; Dukovic-Schultz, S.; Scholl, I.; Schreiber, C.; Sleeman, J. P.
2015. 15th International Biennial Congress of the Metastasis Research Society, Heidelberg, June 28 - July 1, 2014 Clinical and Experimental Metastasis, 32(2015) (Abstract)
Kuch, V.; Krachulec, J.; Obradovich, L.; Hong, J.; Sleeman, J. P.
2015. 15th International Biennial Congress of the Metastasis Research Society, Heidelberg, June 28 - July 1, 2014 Clinical and Experimental Metastasis, 32(2015) (Abstract)
Schmaus, A.; Klusmeier, S.; Bauer, J.; Thiele, W.; Rothley, M.; Quagliata, L.; Dimmler, A.; Jackson, D.; Sleeman, J.
2015. 15th International Biennial Congress of the Metastasis Research Society, Heidelberg, June 28 - July 1, 2014 Clinical and Experimental Metastasis, 32(2015) (Abstract)
Schmaus, A.; Sleeman, J. P.
2015. Glycobiology, 25, 258–268. doi:10.1093/glycob/cwu106
Mudduluru, G.; Abba, M.; Batliner, J.; Patil, N.; Scharp, M.; Lunavat, T. R.; Leupold, J. H.; Oleksiuk, O.; Juraeva, D.; Thiele, W.; Rothley, M.; Benner, A.; Ben-Neriah, Y.; Sleeman, J.; Allgayer, H.
2015. Cancer Research, 75 (15), 3010–3019. doi:10.1158/0008-5472.CAN-15-0997
Seubert, B.; Grünwald, B.; Kobuch, J.; Cui, H.; Schelter, F.; Schaten, S.; Siveke, J. T.; Lim, N. H.; Nagase, H.; Simonavicius, N.; Heikenwalder, M.; Reinheckel, T.; Sleeman, J. P.; Janssen, K. P.; Knolle, P. A.; Krüger, A.
2015. Hepatology, 61, 238–248. doi:10.1002/hep.27378
Hansen, M. T.; Forst, B.; Cremers, N.; Quagliata, L.; Ambartsumian, N.; Grum-Schwensen, B.; Klingelhöfer, J.; Abdul-Al, A.; Herrmann, P.; Osterland, M.; Stein, U.; Nielsen, G. H.; Scherer, P. E.; Lukanidin, E.; Sleeman, J. P.; Grigorian, M.
2015. Oncogene, 34 (4), 424–435. doi:10.1038/onc.2013.568
Augustin, H.; Sleeman, J. P.
2014. Cancer Imaging, 14 (Suppl. 1), O19. doi:10.1186/1470-7330-14-S1-O19
Schmaus, A.; Bauer, J.; Sleeman, J. P.
2014. Cancer and metastasis reviews, 33, 1059–1079. doi:10.1007/s10555-014-9532-2
Schmaus, A.; Klusmeier, S.; Rothley, M.; Dimmler, A.; Sipos, B.; Faller, G.; Thiele, W.; Allgayer, H.; Hohenberger, P.; Post, S.; Sleeman, J. P.
2014. The British journal of cancer, 111, 559–567. doi:10.1038/bjc.2014.332
Orian-Rousseau, V.; Sleeman, J.
2014. Advances in cancer research, 123, 231–254. doi:10.1016/B978-0-12-800092-2.00009-5
Heckmann, D.; Maier, P.; Laufs, S.; Li, L.; Sleeman, J. P.; Trunk, M. J.; Leupold, J. H.; Wenz, F.; Zeller, W. J.; Fruehauf, S.; Allgayer, H.
2014. Clinical cancer research, 20, 604–616. doi:10.1158/1078-0432.CCR-13-0582
Quagliata, L.; Klusmeier, S.; Cremers, N.; Pytowski, B.; Harvey, A.; Pettis, R. J.; Thiele, W.; Sleeman, J. P.
2014. Clinical & experimental metastasis, 31, 351–365. doi:10.1007/s10585-013-9633-2
Fuchs, K.; Hippe, A.; Schmaus, A.; Homey, B.; Sleeman, J. P.; Orian-Rousseau, V.
2013. Cell death & disease, 4, Art. Nr. e819. doi:10.1038/cddis.2013.364
Muppala, S.; Mudduluru, G.; Leupold, J. H.; Buergy, D.; Sleeman, J. P.; Allgayer, H.
2013. PLoS One, 8, e59563/1–12. doi:10.1371/journal.pone.0059563
Schreiber, C.; Kuch, V.; Umansky, V.; Sleeman, J. P.
2013. PLoS One, 8, e57465/1–10. doi:10.1371/journal.pone.0057465
Quero, L.; Klawitter, M.; Schmaus, A.; Rothley, M.; Sleeman, J.; Tiaden, A. N.; Klasen, J.; Boos, N.; Hottiger, M. O.; Wuertz, K.; Richards, P. J.
2013. Arthritis Research and Therapy, 15 (4), R94/1–13. doi:10.1186/ar4274
Thiele, W.; Rothley, M.; Teller, N.; Jung, N.; Bulat, B.; Plaumann, D.; Vanderheiden, S.; Schmaus, A.; Cremers, N.; Göppert, B.; Dimmler, A.; Eschbach, V.; Quagliata, L.; Thaler, S.; Marko, D.; Bräse, S.; Sleeman, J. P.
2013. Carcinogenesis, 34, 2804–2813. doi:10.1093/carcin/bgt291
Kuch, V.; Schreiber, C.; Thiele, W.; Umansky, V.; Sleeman, J. P.
2013. International Journal of Cancer, 132, E94-E105. doi:10.1002/ijc.27785
Sleeman, J. P.; Cady, B.; Pantel, K.
2012. Clinical and Experimental Metastasis, 29, 737–746. doi:10.1007/s10585-012-9489-x
Kaindl, T.; Rieger, H.; Kaschel, L. M.; Engel, U.; Schmaus, A.; Sleeman, J.; Tanaka, M.
2012. PLoS One, 7, e42991/1–10. doi:10.1371/journal.pone.0042991
Sleeman, J. P.
2012. Cancer and Metastasis Reviews, 31, 429–440. doi:10.1007/s10555-012-9373-9
Thaler, S.; Schmidt, M.; Schad, A.; Sleeman, J. P.
2012. Oncogene, 31, 4912–4922. doi:10.1038/onc.2011.658
Neeb, A.; Wallbaum, S.; Novac, N.; Dukovic-Schulze, S.; Scholl, I.; Schreiber, C.; Schlag, P.; Moll, J.; Stein, U.; Sleeman, J. P.
2012. Oncogene, 31, 3796–3806. doi:10.1038/onc.2011.535
Thiele, W.; Krishnan, J.; Rothley, M.; Weih, D.; Plaumann, D.; Kuch, V.; Quagliata, L.; Weich, H. A.; Sleeman, J. P.
2012. Blood, 120, 1899–1907. doi:10.1182/blood-2011-09-376657
Sleeman, J. P.
2012. Seminars in Cancer Biology, 22, 173. doi:10.1016/j.semcancer.2012.03.001
Sleeman, J. P.; Christofori, G.; Fodde, R.; Collard, J. G.; Berx, G.; Decraene, C.; Rüegg, C.
2012. Seminars in Cancer Biology, 22, 174–186. doi:10.1016/j.semcancer.2012.02.007
Lukanidin, E.; Sleeman, J. P.
2012. Seminars in Cancer Biology, 22, 216–225. doi:10.1016/j.semcancer.2012.02.006
Baumann, P.; Thiele, W.; Cremers, N.; Muppala, S.; Krachulec, J.; Diefenbacher, M.; Kassel, O.; Mudduluru, G.; Allgayer, H.; Frame, M.; Sleeman, J. P.
2012. Cellular and Molecular Life Sciences, 69, 435–448. doi:10.1007/s00018-011-0756-9
Kaufmann, L.; Marinescu, G.; Nazarenko, I.; Thiele, W.; Oberle, C.; Sleeman, J.; Blattner, C.
2011. Cell Communication and Signaling, 9, 15. doi:10.1186/1478-811X-9-15
Thanisch, C. D.
2011. Karlsruher Institut für Technologie (KIT)
Schmaus, A.
2011. Karlsruher Institut für Technologie (KIT)
Möller, H. D.; Ralfkjär, U.; Cremers, N.; Frankel, M.; Troelsgaard Pedersen, R.; Klingelhöfer, J.; Yanagisawa, H.; Grigorian, M.; Guldberg, P.; Sleeman, J.; Lukanidin, E.; Ambartsumian, N.
2011. Molecular Cancer Research, 9, 553–563. doi:10.1158/1541-7786.MCR-11-0093
Thiele, W.; Novac, N.; Mink, S.; Schreiber, C.; Plaumann, D.; Fritzmann, J.; Cremers, N.; Rothley, M.; Schwager, C.; Regiert, T.; Huber, P. E.; Stein, U.; Schlag, P.; Moll, J.; Abdollahi, A.; Sleeman, J. P.
2011. Journal of Pathology, 225, 96–105. doi:10.1002/path.2924
Schmitteckert, S.
2011. Karlsruher Institut für Technologie (KIT)
Grau, N.; Sleeman, J. P.
2011. Metastasis and the Tumor Microenvironment : Joint MRS-AACR Conf., Philadelphia, Pa., September 12-15, 2010 Clinical and Experimental Metastasis, 28(2011) (Abstract)
Grau, N.; Sleeman, J. P.
2011. Clinical & experimental metastasis, Metastasis and the Tumor Microenvironment : Joint MRS-AACR Conf., Philadelphia, Pa., September 12-15, 2010, (Abstract), 28, 237
Witte, M. H.; Dellinger, M. T.; McDonald, D. M.; Nathanson, S. D.; Boccardo, F. M.; Campisi, C. C. C.; Sleeman, J. P.; Gershenwald, J. E.
2011. Journal of Surgical Oncology, 103, 489–500. doi:10.1002/jso.21714
Sleeman, J. P.; Nazarenko, I.; Thiele, W.
2011. International Journal of Cancer, 128, 2511–2526. doi:10.1002/ijc.26027
Sleeman, J. P.; Thiery, J. P.
2011. Cell, 145 (1), 162–162.e1. doi:10.1016/j.cell.2011.03.029
Virlouvet, M.; Gartner, M.; Koroniak, K.; Sleeman, J. P.; Bräse, S.
2010. Advanced synthesis & catalysis, 352 (14-15), 2657–2662. doi:10.1002/adsc.201000008
Grau, N.
2010. Karlsruher Institut für Technologie (KIT)
Cremers, N.; Deugnier, M. A.; Sleeman, J.
2010. Cellular and Molecular Life Sciences, 67, 2311–2322. doi:10.1007/s00018-010-0342-6
Sleeman, J. P.; Steeg, P. S.
2010. European Journal of Cancer, 46, 1177–1180. doi:10.1016/j.ejca.2010.02.039
Müller, T.; Stein, U.; Poletti, A.; Garzia, L.; Rothley, M.; Plaumann, D.; Thiele, W.; Bauer, M.; Galasso, A.; Schlag, P.; Pankratz, M.; Zollo, M.; Sleeman, J. P.
2010. Oncogene, 29, 2393–2403. doi:10.1038/onc.2010.6
Häuser, R.
2010. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000020930
Stellberger, T.
2010. Karlsruher Institut für Technologie (KIT). doi:10.5445/IR/1000020743
Gruhl, F. J.
2010. KIT Scientific Publishing. doi:10.5445/KSP/1000019341
Sleeman, J.
2010. Phlebolymphology, 17, 99–107
Gebhardt, C.; Averbeck, M.; Diedenhofen, N.; Willenberg, A.; Anderegg, U.; Sleeman, J. P.; Simon, J. C.
2010. Journal of Investigative Dermatology, 130, 141–49. doi:10.1038/jid.2009.210
Sleeman, J. P.; Thiele, W.
2009. International Journal of Cancer, 125, 2747–56. doi:10.1002/ijc.24702
Grau, N.; Sleeman, J.
2009. The Tumor Vessel Interface : Cellular and Molecular Mechanisms of Tumor Progression and Metastasis, Kloster Seeon, September 19-22, 2009
Steeg, P. S.; Anderson, R. L.; Bar-Eli, M.; Chambers, A. F.; Eccles, S. A.; Hunter, K.; Itoh, K.; Kang, Y.; Matrisian, L. M.; Sleeman, J. P.; Theodorescu, D.; Thompson, E. W.; Welch, D. R.
2009. Clinical Cancer Research, 15, 4529–30. doi:10.1158/1078-0432.CCR-09-1363
Sleeman, J.; Schmid, A.; Thiele, W.
2009. Seminars in Cancer Biology, 19, 285–97. doi:10.1016/j.semcancer.2009.05.005
Teller, N.; Thiele, W.; Boettler, U.; Sleeman, J.; Marko, D.
2009. Molecular Nutrition and Food Research, 53, 1075–83. doi:10.1002/mnfr.200800524
Wallbaum, S.; Grau, N.; Schmid, A.; Frick, K.; Neeb, A.; Sleeman, J. P.
2009. BioTechniques, 46, 433–40. doi:10.2144/000113121
Rothley, M.; Schmid, A.; Thiele, W.; Schacht, V.; Plaumann, D.; Gartner, M.; Yektaoglu, A.; Bruyere, F.; Noel, A.; Giannis, A.; Sleeman, J. P.
2009. International Journal of Cancer, 125, 34–42. doi:10.1002/ijc.24295
Anderegg, U.; Eichenberg, T.; Parthaune, T.; Haiduk, C.; Saalbach, A.; Milkova, L.; Ludwig, A.; Grosche, J.; Averbeck, M.; Gebhardt, C.; Voelcker, V.; Sleeman, J. P.; Simon, J. C.
2009. Journal of Investigative Dermatology, 129, 1471–82. doi:10.1038/jid.2008.323
Teller, N.; Thiele, W.; Marczylo, T. H.; Gescher, A. J.; Boettler, U.; Sleeman, J.; Marko, D.
2009. Journal of Agricultural and Food Chemistry, 57, 3094–3101. doi:10.1021/jf803094a
Rothley, M.
2008. Universität Karlsruhe (TH)
Sleeman, J. P.
2008. Marme, D. [Hrsg.] Tumor Angiogenesis : Basic Mechanisms and Cancer Therapy Berlin [u.a.] : Springer, 2008, 341–50
Bruyere, F.; Melen-Lamalie, L.; Blacher, S.; Roland, G.; Thiry, M.; Moons, L.; Frankenne, F.; Carmeliet, P.; Alitalo, K.; Libert, C.; Sleeman, J. P.; Foidart, J. M.; Noel, A.
2008. Nature Methods, 5, 431–37. doi:10.1038/nmeth.1205
Bruyere, F.; Melen, L.; Blacher, S.; Thiry, M.; Sleeman, J.; Alitalo, K.; Foidart, J. M.; Noel, A.
2008. Annual Meeting of the Belgian Association for Cancer Research, Bruxelles, B, January 20, 2007 Acta Clinica Belgica, 63(2008) (Abstract)
Bruyere, F.; Melen, L.; Blacher, S.; Thiry, M.; Sleeman, J.; Alitalo, K.; Foidart, J. M.; Noel, A.
2008. Acta Clinica Belgica, Annual Meeting of the Belgian Association for Cancer Research, Bruxelles, B, January 20, 2007, (Abstract), 63, 49
Voelcker, V.; Gebhardt, C.; Averbeck, M.; Saalbach, A.; Wolf, V.; Weih, F.; Sleeman, J.; Anderegg, U.; Simon, J.
2007. Experimental dermatology, (2007), 17 (2), 100–107. doi:10.1111/j.1600-0625.2007.00638.x
Thiele, W.
2007. Universität Karlsruhe (TH)
Sleeman, J. P.; Cremers, N.
2007. Clinical and Experimental Metastasis, 24, 707–15. doi:10.1007/s10585-007-9122-6
Eccles, S.; Paon, L.; Sleeman, J.
2007. Clinical and Experimental Metastasis, 24, 619–36. doi:10.1007/s10585-007-9123-5
Berx, G.; Raspe, E.; Cristofori, G.; Thiery, J.; Sleeman, J. P.
2007. Clinical and Experimental Metastasis, 24, 587–97. doi:10.1007/s10585-007-9114-6
Tremmel, M.; Matzke, A.; Albrecht, I.; Christofori, G.; Rothley, M.; Sleeman, J.; Ponta, H.; Orian-Rousseau, V.
2007. 4th Internat.Kloster Seeon Meeting ’Angiogenesis’, Kloster Seeon, September 16-19, 2006
Saalbach, A.; Klein, C.; Sleeman, J.; Sack, U.; Kauer, F.; Gebhardt, C.; Averbeck, M.; Anderegg, U.; Simon, J. C.
2007. Journal of Immunology, 178, 4966–74
Saalbach, A.; Klein, C.; Sleeman, J.; Sack, U.; Kauer, F.; Gebhardt, C.; Averbeck, M.; Anderegg, U.; Simon, J. C.
2007. 34th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF) Freiburg/Breisgau, Germany - March 8-10, 2007 Experimental Dermatology, 16(2007) (abstracts)
Saalbach, A.; Klein, C.; Sleeman, J.; Sack, U.; Kauer, F.; Gebhardt, C.; Averbeck, M.; Anderegg, U.; Simon, J. C.
2007. Experimental Dermatology, 34th Annual Meeting of the Arbeitsgemeinschaft Dermatologische Forschung (ADF) Freiburg/Breisgau, Germany - March 8-10, 2007, (abstracts), 16, 211
Adam, J.
2007. Forschungszentrum Karlsruhe (FZKA). doi:10.5445/IR/1000006656
Averbeck, M.; Gebhardt, C.; Anderegg, U.; Temeer, C.; Sleeman, J. P.; Simon, J. C.
2007. 36th Annual European Society for Dermatological Research (ESDR) Meeting, Paris, F, September 7-9, 2006 Journal of Investigative Dermatology, 126(2006) (Abstract)
Averbeck, M.; Gebhardt, C.; Anderegg, U.; Temeer, C.; Sleeman, J. P.; Simon, J. C.
2007. Journal of Investigative Dermatology, 36th Annual European Society for Dermatological Research (ESDR) Meeting, Paris, F, September 7-9, 2006,(2006) (Abstract), 126, s43
Averbeck, M.; Gebhardt, C.; Anderegg, U.; Temeer, C.; Sleeman, J. P.; Simon, J. C.
2007. Experimental Dermatology, 16, 580–89. doi:10.1111/j.1600-0625.2007.00568.x
Ester, C.
2007. FZKA. doi:10.5445/IR/1000006284
Titz, B.
2006. Universität Karlsruhe (TH). doi:10.5445/IR/1000005334
Sleeman, J. P.
2006. Lymphology, 39 (2), 62–68
Thiele, W.; Sleeman, J. P.
2006. BioPerspectives : From Basic Research to Industrial Production, Wiesbaden, May 10-12, 2005
Thiele, W.; Sleeman, J. P.
2006. Journal of Biotechnology, 124, 224–41. doi:10.1016/j.jbiotec.2006.01.007
Thiery, J. P.; Sleeman, J. P.
2006. Nature Reviews Molecular Cell Biology, 7, 131–42. doi:10.1038/nrm1835
Baumann, P.; Cremers, N.; Kroese, F.; Orend, G.; Chiquet-Ehrismann, R.; Uede, T.; Yagita, H.; Sleeman, J. P.
2005. Cancer Research, 65, 10783–93
Schempp, C. M.; Kiss, J.; Kirkin, V.; Averbeck, M.; Simon-Haarhaus, B.; Kremer, B.; Termeer, C. C.; Sleeman, J.; Simon, J. C.
2005. Planta Medica, 71, 999–1004. doi:10.1055/s-2005-871303
Müller, T.
2005. Universität Karlsruhe (TH). doi:10.5445/IR/1000003663
Scholl, I.
2005. Universität Karlsruhe (TH). doi:10.5445/IR/1000003645
Heilbock, C.
2005. Universität Karlsruhe (TH). doi:10.5445/IR/1000003391
Gartner, M.; Müller, T.; Simon, J. C.; Giannis, A.; Sleeman, J. P.
2005. ChemBioChem, 6, 171–77. doi:10.1002/cbic.200400195
Kirkin, V.; Thiele, W.; Baumann, P.; Mazitschek, R.; Rohde, K.; Felllbrich, G.; Weich, H.; Waltenberger, J.; Giannis, A.; Sleeman, J. P.
2004. International Journal of Cancer, 112, 986–93. doi:10.1002/ijc.20509
Averbeck, M.; Braun, T.; Pfeifer, G.; Sleeman, J.; Dudda, J.; Martin, S. F.; Kremer, B.; Aktories, K.; Simon, J. C.; Termeer, C.
2004. European Journal of Immunology, 34, 2708–19. doi:10.1002/eji.200425355
Mengwasser, J.; Piau, A.; Schlag, P.; Sleeman, J. P.
2004. Oncogene, 23, 7430–35. doi:10.1038/sj.onc.1207987
Bando, H.; Brokelmann, M.; Toi, M.; Alitalo, K.; Sleeman, J. P.; Sipos, B.; Gröne, H. J.; Weich, H. A.
2004. International Journal of Cancer, 111, 184–91. doi:10.1002/ijc.20211
Weich, H. A.; Bando, H.; Brokelmann, M.; Baumann, P.; Toi, M.; Barleon, B.; Alitalo, K.; Sipos, B.; Sleeman, J.
2004. Journal of Immunological Methods, 285, 145–55. doi:10.1016/j.jim.2003.10.015
Baumann, P.; Kroese, F.; Uede, T.; Sleeman, J. P.
2004. 26.Deutscher Krebskongress, Berlin, 27.Februar - 1.März 2004
Fieber, C.; Baumann, P.; Vallon, R.; Termeer, C.; Simon, J. C.; Hofmann, M.; Angel, P.; Herrlich, P.; Sleeman, J. P.
2004. Journal of Cell Science, 117, 359–67. doi:10.1242/jcs.00831
Mengwasser, J.
2003. Universität Karlsruhe (TH). doi:10.5445/IR/1192003
Trappen, P. O. V.; Steele, D.; Lowe, D. G.; Baithun, S.; Beasley, N.; Thiele, W.; Weich, H.; Krishnan, J.; Shepherd, J. H.; Pepper, M. S.; Jackson, D. G.; Sleeman, J. P.; Jacobs, I. J.
2003. The journal of pathology, (2003), 245 (1), 544–554. doi:10.1002/path.1467
Bosserhoff, A. K.; Stoll, R.; Sleeman, J. P.; Bataille, F.; Buettner, R.; Holak, T. A.
2003. Laboratory Investigation, 83, 1583–94
Baumann, P.; Kroese, F.; Orend, G.; Uede, T.; Gröne, J. H.; Schlag, P.; Sleeman, J. P.
2003. Advances in Breast Cancer Research : Genetics, Biology, and Clinical Implications, Huntington Beach, Calif., October 8-12, 2003 8th World Congress on Advances in Oncology and 6th Internat.Symp.on Molecular Medicine, Hersonissos, GR, October 16-18, 2003
Sleeman, J. P.
2003. From Tumour Biology to Clinical Oncology : 31st Meeting of the Internat.Society for Oncodevelopmental Biology and Medicine (ISOBM), Edinburgh, August 30 - September 4, 2003
Bando, H.; Brokelmann, M.; Sipos, B.; Sleeman, J.; Weich, H. A.
2003. 4th Symp.on the Biology of Endothelial Cells, München, July 18-20, 2003
Tille, J. C.; Wang, X.; Lipson, K. E.; Mc Mahon, G.; Ferrara, N.; Zhu, Z.; Hicklin, D. J.; Sleeman, J. P.; Eriksson, U.; Alitalo, K.; Pepper, M. S.
2003. Experimental cell research, 285 (2), 286–298. doi:10.1016/S0014-4827(03)00053-3
Termeer, C.; Averbeck, M.; Hara, H.; Eibel, H.; Herrlich, P.; Sleeman, J.; Simon, J. C.
2003. Immunology, 109, 32–40
Termeer, C.; Sleeman, J. P.; Simon, J. C.
2003. Trends in immunology, 24 (3), 112–114. doi:10.1016/S1471-4906(03)00029-2
Krishnan, J.; Kirkin, V.; Steffen, A.; Hegen, M.; Weih, D.; Tomarev, S.; Wilting, J.; Sleeman, J. P.
2003. Cancer Research, 63, 713–22
Orian-Rousseau, V.; Chen, L.; Sleeman, J. P.; Herrlich, P.; Ponta, H.
2002. Genes and Development, 16, 3074–86
Khaldoyanidi, S.; Karakhanova, S.; Sleeman, J.; Herrlich, P.; Ponta, H.
2002. Blood, 99, 3955–61
Wallach-Dayan, S. B.; Grabovsky, V.; Moll, J.; Sleeman, J.; Herrlich, P.; Alon, R.; Naor, D.
2002. Journal of Cell Science, 114, 3463–77
Krishnan, J.; Kirkin, V.; Mazitschek, R.; Giannis, A.; Sleeman, J. P.
2002. 7th World Congress on Advances in Oncology, 5th Internat.Symp.on Molecular Medicine, Hersonissos, GR, October 10-12, 2002
Mengwasser, J.; Liu, F. T.; Sleeman, J. P.
2002. Glycobiology, 12, 129–34
Göttlicher, M.; Zhu, P.; Werling, U.; Sleeman, J.; Krämer, O.; Heinzel, T.
2002. Nachrichten - Forschungszentrum Karlsruhe, 34, 69–74
Sleeman, J. P.; Baumann, P.; Novac, N.; Thies, W. G.; Nestl, A.; Stein, O. von; Hofmann, M.
2002. Nachrichten - Forschungszentrum Karlsruhe, 34, 31–35
Schempp, C. M.; Kirkin, V.; Simon-Haarhaus, B.; Kersten, A.; Kiss, J.; Termeer, C. C.; Gilb, B.; Kaufmann, T.; Borner, C.; Sleeman, J. P.; Simon, J. C.
2002. Oncogene, 21, 1242–50
Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O. H.; Schimpf, A.; Giavara, S.; Sleeman, J. P.; Lo Coco, F.; Nervi, C.; Pelicci, P. G.; Heinzel, T.
2002. 93rd Annual Meeting of the American Association for Cancer Research, San Francisco, Calif., April 6-10, 2002
Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O. H.; Schimpf, A.; Giavara, S.; Sleeman, J. P.; Lo Coco, F.; Nervi, C.; Pelicci, P. G.; Heinzel, T.
2002. 43.Frühjahrstagung der Deutschen Gesellschaft für experimentelle und klinische Pharmakologie und Toxikologie (DGPT), Mainz, 12.-14.März 2002 Naunyn-Schmiedebergs Archives of Pharmacology, 365(2002) No.1 Teil Suppl., (Abstract)
Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J. C.
2002. Journal of Experimental Medicine, 195, 99–111. doi:10.1084/jem.20001858
Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O. H.; Schimpf, A.; Giavara, S.; Sleeman, J. P.; Lo Coco, F.; Nervi, C.; Pelicci, G.; Heinzel, T.
2002. The EMBO Journal, (2001), 20, 6969–78
Kirkin, V.; Mazitschek, R.; Krishnan, J.; Steffen, A.; Waltenberger, J.; Pepper, M. S.; Giannis, A.; Sleeman, J. P.
2001. European Journal of Biochemistry, 268, 5530–40
Sleeman, J. P.; Krishnan, J.; Kirkin, V.; Baumann, P.
2001. Microscopy Research and Technique, 55, 61–69
Papoutsi, M.; Sleeman, J. P.; Wilting, J.
2001. Microscopy Research and Technique, 55, 100–07
Mengwasser, J.; Sleeman, J. P.
2001. Glycobiology, 11, 441–49
Sleeman, J. P.; Novac, N.; Thies, W. G.; Nestl, A.; Stein, O. von; Hofmann, M.
2001. Lippert, B.M. [Hrsg.] Metastases in Head and Neck Cancer : Proc.of the 2nd Internat.Symp., Marburg, January 25-27, 2001 Marburg : Tectum Verl., 2001
Sleeman, J. P.; Novac, N.; Thies, W. G.; Nestl, A.; Stein, O. von; Hofmann, M.
2001. Lippert, B.M. [Hrsg.] Metastases in Head and Neck Cancer : Proc.of the 2nd Internat.Symp., Marburg, January 25-27, 2001 Marburg : Tectum Verl., 2001, 93–97
Ahrens, T.; Sleeman, J. P.; Schempp, C. M.; Howells, N.; Hofmann, M.; Ponta, H.; Herrlich, P.; Simon, J. C.
2001. Oncogene, 20, 3399–3408
Sleeman, J. P.; Thies, W. G.; Nestl, A.; Stein, O. von; Hofmann, M.
2001. Munz, D.L. [Hrsg.] The Sentinel Lymph Node Concept in Oncology : Facts and Fiction ; Internat.Forum Nuclear Medicine, Charite Berlin München [u.a.] : Zuckschwerdt Verl., 2001, 13–25
Nestl, A.; Stein, O. D. von; Zatloukal, K.; Thies, W. G.; Herrlich, P.; Hofmann, M.; Sleeman, J. P.
2001. Cancer Research, 61, 1569–77
Sleeman, J. P.
2000. Fak. f. Bio- und Geowissenschaften, Habil.-Schr. v. 28.6.2000
Novac, N.; Müller, T.; Nestl, A.; Stein, O. von; Thies, W.; Zatloukal, K.; Kröse, F.; Moll, J.; Gröne, H. J.; Herrlich, P.; Hofmann, M.; Sleeman, J. P.
2000. 8th Internat.Congress of the Metastasis Research Society, London, September 24-27, 2000
Krishnan, J.; Kirkin, V.; Baumann, P.; Katzenberger, J.; Waltenberger, J.; Weich, H.; Sleeman, J.
2000. Internat.Symp.of the German Priority Research Program SPP1069: Angiogenesis, Kloster Seeon, 1.-4.Oktober 2000
Herrlich, P.; Morrison, H.; Sleeman, J.; Orian-Rousseau, V.; König, H.; Weg-Remers, S.; Ponta, H.
2000. Colorectal Cancer: New Aspects of Molecular Biology and Immunology and Their Clinical Applications, Berlin, October 8-9, 2000 Annals of the New York Academy of Sciences, 910(2000)
Herrlich, P.; Morrison, H.; Sleeman, J.; Orian-Rousseau, V.; König, H.; Weg-Remers, S.; Ponta, H.
2000. Annals of the New York Academy of Sciences, Colorectal Cancer: New Aspects of Molecular Biology and Immunology and Their Clinical Applications, Berlin, October 8-9, 2000, 910, 106–20
Hebbard, L.; Steffen, A.; Zawadzki, V.; Fieber, C.; Howells, N.; Moll, J.; Ponta, H.; Hofmann, M.; Sleeman, J.
2000. Journal of Cell Science, 113, 2619–30
Sleeman, J. P.
2000. Habilitation, Universität Karlsruhe 2000
Sleeman, J. P.
2000. Recent Results in Cancer Research, 157, 55–81
Sherman, L.; Sleeman, J. P.; Hennigan, R. F.; Herrlich, P.; Ratner, N.
2000. Oncogene, (1999), 18, 6692–99
Novac, N.; Moll, J.; Sleeman, J. P.
2000. 24.Jahrestagung der Deutschen Gesellschaft für Zellbiologie, Karlsruhe, 26.-30.März 2000
Kirkin, V.; Sleeman, J. P.
2000. 24.Jahrestagung der Deutschen Gesellschaft für Zellbiologie, Karlsruhe, 26.-30.März 2000
Krishnan, J.; Steffen, A.; Mandriotta, S.; Pepper, M.; Sleeman, J. P.
1999. 7.KBF-Symp., Common Mechanisms in Development and Disease, Karlsruhe, September 8-10, 1999
Sleeman, J. P.; Kim, U.; Lependu, J.; Howells, N.; Coquerelle, T.; Ponta, H.; Herrlich, P.
1999. Oncogene, 18, 4485–94
Fieber, C.; Plug, R.; Sleeman, J.; Dall, P.; Ponta, H.; Hofmann, M.
1999. Gene, 226, 41–50
Weiss, J. M.; Renkl, A. C.; Sleeman, J.; Dittmar, H.; Termeer, C. C.; Taxis, S.; Howells, N.; Schöpf, E.; Ponta, H.; Herrlich, P.; Simon, J. C.
1999. Cell Adhesion and Communication, (1998), 6, 157–60
Katagiri, Y. U.; Sleeman, J.; Fujii, H.; Herrlich, P.; Hotta, H.; Tanaka, K.; Chikuma, S.; Yagita, H.; Okumura, K.; Murakami, M.; Saiki, I.; Chambers, A. F.; Uede, T.
1999. Cancer Research, 59, 219–26
Sleeman, J. P.; Steffen, A.; Mandriotta, A.; Krishnan, J.; Pepper, M.
1998. 5th Franz-Vollhard-Symp.’Twigs and Branches-Tube Formation and Branching in the Cardiovascular System’, Groß Dölln, 3.-6.September 1998
Sleeman, J. P.; Kim, U.; Lependu, J.; Howells, N.; Ponta, H.; Herrlich, P.
1998. 7th Internat.Congress of the Metastasis Research Society, San Diego, Calif., October 7-10, 1998
Ponta, H.; Sleeman, J.; Sherman, L.; Moll, J.; Herrlich, P.
1998. Congress ’Biological Therapy of Cancer’, München, June 11-14, 1997 Internat.Workshop on Surgery and Critical Care Cellular and Molecular Mechanisms, Bonn, March 5-7, 1998
Hofmann, M.; Assmann, V.; Fieber, C.; Sleeman, J.; Moll, J.; Ponta, H.; Hart, I. A.; Herrlich, P.
1998. Cell, 95, 591–93
Herrlich, P.; Sleeman, J.; Wainwright, D.; König, H.; Sherman, L.; Hilberg, F.; Ponta, H.
1998. Cell Adhesion and Communication, 6, 141–47
Sleeman, J. P.; Rahmsdorf, U.; Steffen, A.; Ponta, H.; Herrlich, P.
1998. European Journal of Biochemistry, 255, 74–80
Moll, J.; Khaldoyanidi, S.; Sleeman, J. P.; Achtnich, M.; Preuss, I.; Ponta, H.; Herrlich, P.
1998. Journal of Clinical Investigation, 102, 1024–34
Hofmann, M.; Fieber, C.; Assmann, V.; Göttlicher, M.; Sleeman, J.; Plug, R.; Howells, N.; Stein, O. von; Ponta, H.; Herrlich, P.
1998. Journal of Cell Science, 111, 1673–84
Gao, A. C.; Lou, W.; Sleeman, J. P.; Isaacs, J. T.
1998. Cancer Research, 58, 2350–52
Amaravadi, L. S.; Neff, A. W.; Sleeman, J. P.; Smith, R. C.
1998. Developmental Biology, (1997), 192, 392–404
Herrlich, P.; Sleeman, J.; Sherman, L.; Wainwright, D.; Ponta, H.
1997. European Science Foundation Research Conf.’Molecular Biology of Cellular Interactions: Cell Adhesion Molecules and Receptor Cross-Talk’, Granada, E, October 31 - November 5, 1997
Herrlich, P.; Sleeman, J.; Sherman, L.; Wainwright, D.; Ponta, H.
1997. 2nd Amsterdam ’Zoo’ Meeting - Cell Adhesion and Migration in Inflammation and Cancer, Amsterdam, NL, October 22-25, 1997
Sherman, L.; Sleeman, J.; Hennigan, R. F.; Ratner, N.; Ponta, H.; Herrlich, P.
1997. 37th Annual Meeting of the American Society for Cell Biology, Washington, D.C.; December 13-17, 1997
Herrlich, P.; Ponta, H.; Sleeman, J.; Stein, O. von; Wainwright, D.
1997. 7th Charles Heidelberger Meeting, Cancer: Genesis - Detection - Therapy, Ulm, July 2-5, 1997
Weiss, J. M.; Sleeman, J.; Dittmar, H.; Termeer, C. C.; Renkl, A. C.; Howells, N.; Ponta, H.; Herrlich, P.; Simon, J. C.
1997. 2nd Amsterdam ’Zoo’ Meeting - Cell Adhesion and Migration in Inflammation and Cancer, Amsterdam, NL, October 22-25, 1997
Ponta, H.; Sleeman, J.; Sherman, L.; Moll, J.; Herrlich, P.
1997. American Association for Cancer Research (AACR) Special Conf.: Cell Signaling and Cancer Treatment, Telfs, A, February 23-28, 1997
Herrlich, P.; Sleeman, J.; Moll, J.; Sherman, L.
1997. 5.Deutsch-Koreanisches Symp., Würzburg, 23.-27.Juli 1997
Sleeman, J.
1997. Nachrichten - Forschungszentrum Karlsruhe, 29, 241–43
Sleeman, J.; Kondo, K.; Moll, J.; Ponta, H.; Herrlich, P.
1997. The Journal of Biological Chemistry, 272, 31837–44
Weiss, J. M.; Sleeman, J.; Renkl, A. C.; Dittmar, H.; Termeer, C. C.; Taxis, S.; Howells, N.; Hofmann, M.; Köhler, G.; Schöpf, E.; Ponta, H.; Herrlich, P.; Simon, J. C.
1997. The Journal of Cell Biology, 137, 1137–47
Sleeman, J.
1996. Robert Steel Foundation Symp., Memorial Sloan Ketterin Cancer Center, New York, N.Y., September 18-20, 1996
Herrlich, P.; Sleeman, J.; Sherman, L.; Ponta, H.; Moll, J.
1996. Internat.Summer School on Mechanisms in Eukaryotic Gene Regulation, Spetses, GR, September 2-12, 1996
Hekele, A.; Sleeman, J.; Sherman, L.; Hofmann, M.; Herrlich, P.
1996. 5th Internat.Expert Forum of Immunotherapy and Gene Therapy, Jerusalem, IL, June 4-7, 1996
Sleeman, J.; Arming, S.; Moll, J.; Herrlich, P.; Ponta, H.
1996. 12th Internat.Conf.on Lymphoid Tissues and Germinal Centers in Immune Reactions, Graz, A, July 1-5, 1996
Moll, J.; Sleeman, J.; Khaldoyanidi, S.; Ponta, H.; Herrlich, P.
1996. 10th GIF Meeting on Cell Biology of Protein Interactions, Signalling Translocation and Degradation, Dead Sea, IL, March 10-15, 1996
Sherman, L.; Sleeman, J.; Dall, P.; Hekele, A.; Moll, J.; Ponta, H.; Herrlich, P.
1996. Current Topics in Microbiology and Immunology, Günthert, U. [Hrsg.] Attempts to Understand MetastasisFormation I Berlin [u.a.] : Springer, 1996 ( ; 213/I), 249–69
Sleeman, J.; Rudy, W.; Hofmann, M.; Moll, J.; Herrlich, P.; Ponta, H.
1996. The Journal of Cell Biology, 135, 1139–50
Wainwright, D.; Sherman, L.; Sleeman, J.; Ponta, J.; Herrlich, P.
1996. Annals of the New York Academy of Sciences, 785, 345–49
Sleeman, J.; Arming, S.; Moll, J.; Hekele, A.; Rudy, W.; Sherman, L.; Kreil, G.; Ponta, H.; Herrlich, P.
1996. Cancer Research, 56, 3134–41
Moll, J.; Sleeman, J.; Kondo, K.; Hekele, A.; Plug, R.; Sherman, L.; Ponta, H.; Herrlich, P.; Schmidt, A.; Zöller, M.; Horst, E.; Pals, S. T.
1995. Proc.of the 24th Internat.Symp.of the Princess Takamatsu Cancer Research Fund Princeton Scientific Publ.Co., 1994
Sleeman, J.; Moll, J.; Sherman, L.; Dall, P.; Pals, S. T.; Ponta, H.; Herrlich, P.
1995. Marsh, J. [Hrsg.] Cell Adhesion and Human Desease : Proc.of a Symp., London, May 17-19, 1994 Chichester [u.a.] : John Wiley and Sons, 1995 (Ciba Foundation Symp. ; 189)
Sleeman, J.; Vallon, R.; König, H.; Sherman, L.; Ponta, H.; Herrlich, P.
1995. 24th European Symp.on Calcified Tissues, Aarhus, DK, May 27-30, 1995
Moll, J.; Sleeman, J.; Kondo, K.; Hekele, A.; Plug, R.; Sherman, L.; Ponta, H.; Herrlich, P.; Schmidt, A.; Zöller, M.; Horst, E.; Pals, S. T.
1995. Proc.of the 24th Internat.Symp.of the Princess Takamatsu Cancer Research Fund Princeton Scientific Publ.Co., 1994, 142–51
Sleeman, J.; Moll, J.; Sherman, L.; Dall, P.; Pals, S. T.; Ponta, H.; Herrlich, P.
1995. Marsh, J. [Hrsg.] Cell Adhesion and Human Desease : Proc.of a Symp., London, May 17-19, 1994 Chichester [u.a.] : John Wiley and Sons, 1995 (Ciba Foundation Symp. ; 189), 142–56
Ponta, H.; Sleeman, J.; Dall, P.; Moll, J.; Sherman, L.; Herrlich, P.
1995. Invasion and Metastasis, 14(1994/95) -6, (1), 82–86
Hudson, D. L.; Sleeman, J.; Watt, F. M.
1995. Journal of Cell Science, 108, 1959–70
Ponta, H.; Dall, P.; Hekele, A.; Heider, K. H.; Sleeman, J.; Pals, S. T.; Herrlich, P.
1994. 13th Meeting of the European Association for Cancer Research, Berlin, September 25-28, 1994
Herrlich, P.; Ponta, H.; Sleeman, J.; Hekele, A.; Dall, P.; Pals, S. T.
1994. 6th EORTC Breast Cancer Working Conf., Amsterdam, NL, September 6-9, 1994
Moll, J.; Sleeman, J.; Sherman, L.; Plug, G.; Putten, H. van der; Ponta, H.; Herrlich, P.
1994. EMBL Conf.’Oncogenes and Growth Control’ Heidelberg, April 18-21, 1994
Sherman, L.; Sleeman, J.; Herrlich, P.; Ponta, H.
1994. Current Opinion in Cell Biology, 6, 726–33
Ponta, H.; Sleeman, J.; Herrlich, P.
1994. Biochimica et Biophysica Acta, 1198, 1–10
Moll, J.; Heider, K. H.; Sleeman, J.; Kaufmann, M.; Zoeller, M.; Adolf, G. R.; Arch, R.; Rudy, W.; Ponta, H.; Herrlich, P.
1993. Annual Meeting of the Society for Histochemistry, Gargellen, A, September 29 - October 2, 1993
Sleeman, J.; Kondo, K.; Moll, J.; Zoeller, M.; Arch, R.; Rudy, W.; Ponta, H.; Herrlich, P.
1993. XI. Congresso ABCD (Cell Biology and Development Association), Milano, I, September 16-19, 1993
Heider, K. H.; Kaufmann, M.; Sleeman, J.; Arch, R.; Zoeller, M.; Ponta, H.; Herrlich, P.
1993. 24th Internat.Symp.of the Princess Takamatsu Cancer Research Fund, Tokyo, J, November 9-11, 1993
Kaufmann, M.; Heider, K. H.; Sinn, H. P.; Sleeman, J.; Kondo, K.; Ponta, H.; Pals, S. T.; Zoeller, M.; Herrlich, P.
1993. Cell Adhesion Meeting ’Cell Adhesion - Regulation and Clinical Prospects’, Amsterdam, NL, October 13-16, 1993
Herrlich, P.; Rudy, W.; Hofmann, M.; Arch, R.; Zoeller, M.; Zawadzki, V.; Toelg, C.; Hekele, A.; Koopman, G.; Pals, S. T.; Heider, K. H.; Sleeman, J.; Ponta, H.
1993. Hemler, M.E. [Hrsg.] Cell Adhesion Molecules New York [u.a.] : Plenum, 1993, 265–88
Eibl, R. H.; Arch, R.; Toelg, C.; Zawadzki, V.; Heider, K. H.; Rudy, W.; Wirth, K.; Matzku, S.; Pals, S.; Moll, J.; Sleeman, J. P.; Hofman, M.; Zoeller, M.; Ponta, H.; Herrlich, P.
1992. EMBO Workshop on Cell-Cell Interactions in Leukocyte Homing and Differentiation, Basel, CH, September 1-4, 1992
