Skeletal Muscle Differentiation
Research interests
Skeletal muscle is an extremely plastic tissue which can adapt to the changing demands of the organism. A fascinating property of skeletal muscle is its amazing capacity to repair and regenerate. However, this capacity declines with age and is affected in various pathological conditions such as certain myopathies.
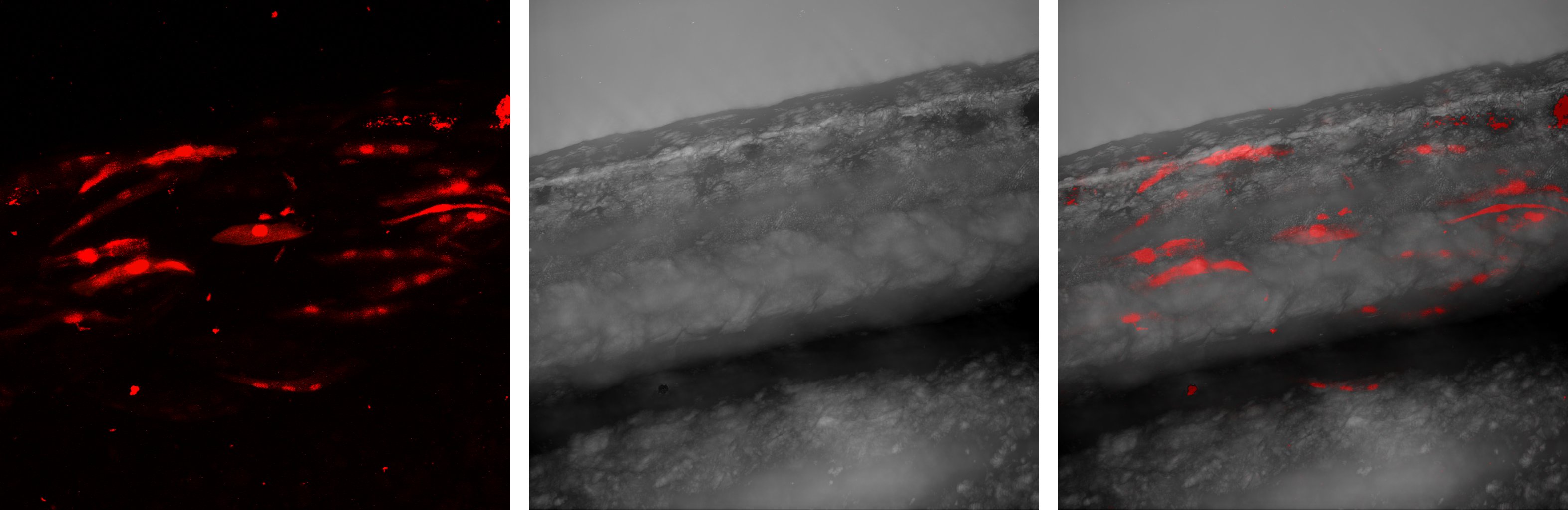
Muscle regeneration relies on the process of post-natal myogenesis, which involves adult, resident stem cells, the so-called satellite cells. Upon muscle damage, these normally quiescent cells enter a differentiation programme, which takes them through well-defined, tightly regulated differentiation stages. Activated satellite cells first give rise to a population of proliferating transit amplifying cells, the so-called myoblasts. After a certain number of cell cycles, myoblasts stop proliferating and differentiate into committed precursor cells, the myocytes. Myocytes then fuse together to form new immature myofibres, or to damaged fibres to repair injured muscles. Finally, the maturation stage involves in particular a regulation of the myofibre size.
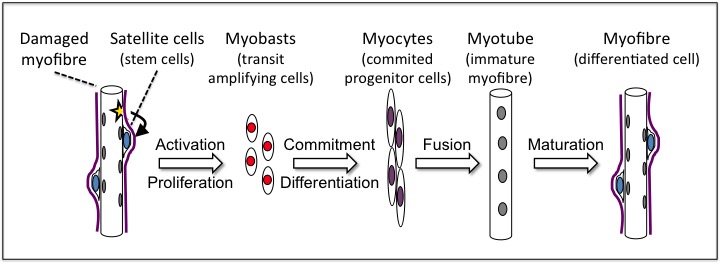
The different stages of adult myogenesis
Harnessing the mechanisms of myogenesis holds great promise for future therapeutic approaches to treat muscle wasting. However, a prerequisite is a deeper understanding of the molecular mechanisms involved, in particular the specific signalling pathways and genetic programmes that ensure a proper timing of cell fate decision at the different stages of myogenesis.
Current projects
- Regulation and function of the transcriptional regulator nTRIP6
- Role of translational regulation in myogenesis
- Regulation of myofibre size in the developing zebrafish embryo
- Development of molecular tools (collaboration with the groups of Olalla Vázquez, Philipps Universität Marburg, and of Barbara Di Ventura, BIOSS, University of Freiburg)
- Zebrafish models of human genetic diseases (collaboration with Hélène Dolfus, Laboratoire de Génétique Médicale, Université de Strasbourg)
Dr. Olivier Kassel, PhD
- Group leader
- Group: Kassel
- Room: 242
CN 0319 - Phone: +49 721 608-23911
- olivier kassel ∂does-not-exist.kit edu
- ORCID
Karlsruhe Institute of Technology (KIT)
Campus North
Institute of Biological and Chemical Systems (IBCS)
Building 319
Hermann-von-Helmholtz-Platz 1
76344 Eggenstein-Leopoldshafen
Germany
Publications
Kröll-Hermi, A.; Stoetzel, C.; Etard, C.; Halabelian, L.; Schaefer, E.; Scheidecker, S.; Kahrizi, K.; Payman, J.; Geoffroy, V.; Prasad, M.; Obringer, C.; Ruch, L.; Girard, A.; Zeng, H.; Li, F.; Plassard, D.; Keime, C.; Mattioli, F.; Feger, C.; Piton, A.; Fujita, A.; Matsumoto, N.; Castro, M. A. A.; Ae, K. C.; Ruaud, L.; Levy, J.; Dozières, B.; Tabet, A.-C.; Wentzensen, I. M.; Santiago-Sim, T.; Yusupov, R.; Tveten, K.; Smeland, M. F.; Alkhunaizi, E.; Cowing, G.; Li, C.; Wortmann, S. B.; Feichtinger, R. G.; Mayr, J. A.; Gonorazky, H.; Jing, G.; Wang, X.; Wang, J.; Bierhals, T.; Grinstein, L.; Herget, T.; Ruiz, A.; Gabau, E.; Kampmeier, A.; Kassel, O.; et al.
2025. The American Journal of Human Genetics, 112 (12), 2943–2960. doi:10.1016/j.ajhg.2025.10.014
Siauciuinate, R.; Etard, C.; Koehler, A.; Pisanty, O.; Otto, M.; Dickmeis, T.; Kassel, O.; Gothilf, Y.; Foulkes, N. S.; Vallone, D.
2025. Laboratory Animals, 59 (6), 664–675. doi:10.1177/00236772251351087
Scheidecker, S.; Bär, S.; Kröll-Hermi, A.; Delvallée, C.; Rinaldi, B.; Korpioja, A.; Geoffroy, V.; Schaefer, E.; Secula, S.; Jaeger, C.; Stoetzel, C.; Kassel, O.; Straehle, U.; Bertoli-Avella, A.; Zonic, E.; Lamouche, J.-B.; Zanlonghi, X.; Etard, C.; Muller, J.; Rahikkala, E.; Friant, S.; Dollfus, H.
2025. European Journal of Human Genetics, 33 (11), 1432–1441. doi:10.1038/s41431-025-01803-2
Fettig, R.; Gonda, Z.; Walter, N.; Sallmann, P.; Thanisch, C.; Winter, M.; Bauer, S.; Zhang, L.; Linden, G.; Litfin, M.; Khamanaeva, M.; Storm, S.; Münzing, C.; Etard, C.; Armant, O.; Vázquez, O.; Kassel, O.
2025, February
Fettig, R.; Gonda, Z.; Walter, N.; Sallmann, P.; Thanisch, C.; Winter, M.; Bauer, S.; Zhang, L.; Linden, G.; Litfin, M.; Khamanaeva, M.; Storm, S.; Münzing, C.; Etard, C.; Armant, O.; Vázquez, O.; Kassel, O.
2025. EMBO Reports, 1–24. doi:10.1038/s44319-025-00390-z
Shukla, S.; Haenold, R.; Urbánek, P.; Frappart, L.; Monajembashi, S.; Grigaravicius, P.; Nagel, S.; Min, W. K.; Tapias, A.; Kassel, O.; Heuer, H.; Wang, Z.-Q.; Ploubidou, A.; Herrlich, P.
2021. Nature Communications, 12 (1), Art.-Nr.: 5887. doi:10.1038/s41467-021-26057-6
Norizadeh Abbariki, T.; Gonda, Z.; Kemler, D.; Urbanek, P.; Wagner, T.; Litfin, M.; Wang, Z.-Q.; Herrlich, P.; Kassel, O.
2021. Scientific Reports, 11 (1), Art.Nr. 12904. doi:10.1038/s41598-021-92331-8
Molle, E.; Le, D.; Norizadeh Abbariki, T.; Akdemir, M. S.; Takamiya, M.; Miceli, E.; Kassel, O.; Delaittre, G.
2019. ChemPhotoChem, 3 (11), 1084–1089. doi:10.1002/cptc.201900216
Angelin, A.; Kassel, O.; Rastegar, S.; Strähle, U.; Niemeyer, C. M.
2017. ChemistryOpen, 6 (1), 33–39. doi:10.1002/open.201600153
Kemler, D.; Dahley, O.; Roßwag, S.; Litfin, M.; Kassel, O.
2016. Scientific reports, 6, Art. Nr. 27746. doi:10.1038/srep27746
Chakraborty, A.; Diefenbacher, M. E.; Mylona, A.; Kassel, O.; Behrens, A.
2015. Nature Communications, 6, 6782/1–12. doi:10.1038/ncomms7782
Diefenbacher, M. E.; Reich, D.; Dahley, O.; Kemler, D.; Litfin, M.; Herrlich, P.; Kassel, O.
2014. PLoS ONE, 9 (5), e97549. doi:10.1371/journal.pone.0097549
Röder, I. V.; Strack, S.; Reischl, M.; Dahley, O.; Khan, M. M.; Kassel, O.; Zaccolo, M.; Rudolf, R.
2012. PLoS One, 7 (7), e40860/1–10. doi:10.1371/journal.pone.0040860
Baumann, P.; Thiele, W.; Cremers, N.; Muppala, S.; Krachulec, J.; Diefenbacher, M.; Kassel, O.; Mudduluru, G.; Allgayer, H.; Frame, M.; Sleeman, J. P.
2012. Cellular and Molecular Life Sciences, 69, 435–448. doi:10.1007/s00018-011-0756-9
Diefenbacher, M. E.; Litfin, M.; Herrlich, P.; Kassel, O.
2010. Molecular and Cellular Endocrinology, 320, 58–66. doi:10.1016/j.mce.2010.02.010
Wadhwani, P.; Bürck, J.; Strandberg, E.; Witter, R.; Mink, C.; Afonin, S.; Ieronimo, M.; Diefenbacher, M.; Kassel, O.; Ulrich, A. S.
2008. 8th German Peptide Symp., Heidelberg, March 14-17, 2007 2nd Workshop on Biophysics of Membrane-Active Peptides, Lisboa, P, April 1-4, 2007 Book of Abstracts
Mappes, T.; Lenhert, S.; Kassel, O.; Vannahme, C.; Schelb, M.; Mohr, J.
2008. Digest of the IEEE/LEOS Summer Topicals, Acapulco, MEX, July 21-23, 2008 Publ.online Piscataway, N.J. : IEEE, 2008 DOI:10.1109/LEOSST.2008.4590566, IEEE/LEOS Summer Topical Meetings (2008), Acapulco de Juárez, Mexico, July 21–23, 2008
Mappes, T.; Lenhert, S.; Kassel, O.; Vannahme, C.; Schelb, M.; Mohr, J.
2008. 2008 Digest of the IEEE/LEOS Summer Topical Meetings, Digest of the IEEE/LEOS Summer Topicals, Acapulco, MEX, July 21-23, 2008 Publ.online Piscataway, N.J. : IEEE, 2008, 215–216, Institute of Electrical and Electronics Engineers (IEEE). doi:10.1109/LEOSST.2008.4590566
Diefenbacher, M.; Sekula, S.; Heilbock, C.; Maier, J. V.; Litfin, M.; Dam, H. van; Castellazzi, M.; Herrlich, P.; Kassel, O.
2008. Molecular Endocrinology, 22, 1767–80. doi:10.1210/me.2007-0574
Wadhwani, P.; Bürck, J.; Witter, R.; Strandberg, E.; Mink, C.; Afonin, S.; Ieronimo, M.; Diefenbacher, M.; Kassel, O.; Ulrich, A. S.
2007. 20th American Peptide Society Symp., Montreal, CDN, June 26-30, 2007 Peptide Science, 88(2007) Special Issue 4, (Abstract)
Wadhwani, P.; Bürck, J.; Witter, R.; Strandberg, E.; Mink, C.; Afonin, S.; Ieronimo, M.; Diefenbacher, M.; Kassel, O.; Ulrich, A. S.
2007. 20th American Peptide Society Symp., Montreal, CDN, June 26-30, 2007 Peptide Science, 88(2007) Special Issue 4, (Abstract)
Wadhwani, P.; Bürck, J.; Witter, R.; Strandberg, E.; Mink, C.; Afonin, S.; Ieronimo, M.; Diefenbacher, M.; Kassel, O.; Ulrich, A. S.
2007. Peptide Science, 20th American Peptide Society Symp., Montreal, CDN, June 26-30, 2007, Special Issue 4, (Abstract), 88, 585
Mink, C.; Diefenbacher, M.; Ieronimo, M.; Tiltak, D.; Wadhwani, P.; Kassel, O.; Ulrich, A. S.
2007. CFN Summer School on Nanobiology, Bad Herrenalb, August 20-23, 2007
Mink, C.; Diefenbacher, M.; Ieronimo, M.; Tiltak, D.; Wadhwani, P.; Kassel, O.; Ulrich, A. S.
2007. 9th Internat.Symp. Solid Phase Synthesis, Norwich, GB, August 27 - September 1, 2007
Kassel, O.; Herrlich, P.
2007. Molecular and Cellular Endocrinology, 275, 13–29. doi:10.1016/j.mce.2007.07.003
Kassel, O.; Schneider, S.; Heilbock, C.; Litfin, M.; Göttlicher, M.; Herrlich, P.
2004. Genes and Development, 18, 2518–2528. doi:10.1101/gad.322404
Silva, C. A. da; Kassel, O.; Lebouquin, R.; Lacroix, E. J.; Frossard, N.
2004. British Journal of Pharmacology, 141, 75–84. doi:10.1038/sj.bjp.0705598
Silva, C. A. da; Heilbock, C.; Kassel, O.; Frossard, N.
2003. FASEB Journal, No.13, 17, U310-U334. doi:10.1096/fj.03-0136fje
Sancono, A.; Kassel, O.; Maier, J.; Hesslinger, C.; Cato, A. C. B.
2003. 1st ERS Lung Science Conf., Taormina, I, March 28-30, 2003 FEBS Young Scientist Forum, Bruxelles, B, July 1-3, 2003 European Respiratory Journal, 22(2003) Suppl., S-41S
Sancono, A.; Kassel, O.; Maier, J.; Hesslinger, C.; Cato, A. C. B.
2003. European Respiratory Journal, 1st ERS Lung Science Conf., Taormina, I, March 28-30, 2003 FEBS Young Scientist Forum, Bruxelles, B, July 1-3, 2003, Suppl., S-41S, 22, 40
Kassel, O.; Cato, A. C. B.
2002. Cato, A.C.B. [Hrsg.] Recent Advances in Glucocorticoid Receptor Action Berlin [u.a.] : Springer, 2002 (Ernst Schering Research Foundation Workshop ; 40), 153–76
Kassel, O.; Sancono, A.; Krätzschmar, J.; Kreft, B.; Cato, A. C. B.
2002. Keystone Symp.’Protein Phosphorylation and Mechanisms of Cellular Regulation (X2)’, Taos, N.M., March 5-10, 2002
Kassel, O.; Sancono, A.; Krätzschmar, J.; Kreft, B.; Stassen, M.; Cato, A. C. B.
2001. The EMBO Journal, (2001), 20 (24), 7108–7116. doi:10.1093/emboj/20.24.7108
Experimental approaches
To address our questions, we use a set of complementary approaches:
Myoblasts in culture (cell line and mouse primary cells)
This approach enables us to recapitulate certain aspects of myogenesis in vitro, in particular to dissect the dynamics of signalling pathways and gene expression during differentiation. We use a large palette of molecular biology, cell biology and chemical biology tools and methods.
Isolated myofibres in culture
Upon isolation from mouse or zebrafish muscles, myofibres retain the attached satellite cells and can be cultured for days. This ex vivo approach allows the study of myogenesis in a context closer to the in vivo situation.
Animal models
We use zebrafish as a model organism to study muscle homeostasis in the context of the whole organism.